Open Journal of Pediatrics and Child Health
A retrospective claims analysis of US pediatricians prescribing practices for the treatment of Molluscum Contagiosum
Martina Cartwright1, Tomoko Maeda-Chubachi1*, Carolyn Enloe1 and Stephen Stripling2
2Coastal Pediatric Associates, Charleston, SC, USA
Cite this as
Cartwright M, Maeda-Chubachi T, Enloe C, Stripling S (2024) A retrospective claims analysis of US pediatricians prescribing practices for the treatment of Molluscum Contagiosum. Open J Pediatr Child Health 9(1): 045-049. DOI: 10.17352/ojpch.000057Copyright
© 2024 Cartwright M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Introduction: Molluscum Contagiosum (MC), a common viral skin infection affects mostly children, is caused by Molluscipoxvirus. MC is characterized by flesh-colored, round, dome-shaped, umbilicated bumps. Pediatricians are often the first to diagnose MC.
Objective: To examine US pediatrician’s MC treatment management using claims data.
Methods: Syneos used the Compile Claims database to collect all medical and pharmacy claims for pediatric patients diagnosed with molluscum (ICD10 Code B081) in 2019-2020. Pediatric patients with a Molluscum (B081) claim from a pediatrician practice were calculated along with patients prescribed a prescription by the same pediatrician within 30 days of a molluscum claim. Practitioners were categorized as “wait and see,” “treater” or “referrer.” Frequency data were captured.
Results: From 2019-2020, 260,788 molluscum patients ≤ 19 years were diagnosed by 56,026 healthcare providers. Pediatricians were responsible for a majority of molluscum diagnoses: 72% of patients (187,228) diagnosed by 35,017 pediatricians. Most pediatricians initially managed MC with a “wait and see” approach (91%). If a “wait and see” pediatrician initiated treatment, half managed by prescription, whereas “treaters” used in-office procedures (81%) and prescriptions (23%). Mupirocin (45%) and triamcinolone (23%) were preferred prescriptions.
Conclusion: Mupirocin and triamcinolone were likely used to treat signs of inflammation indicative of MC resolution known as Beginning of the End (BOTE) sign. Neither are FDA approved for MC nor antiviral; mupirocin use without secondary bacterial infection may contribute to resistance. Newly approved MC indicated therapies should be considered and a better understanding of BOTE is essential to distinguish MC resolution from secondary infection.
Abbreviations
FDA: Food and Drug Administration; HCP: Healthcare Provider; IDN: Integrated Delivery Network; ICD: International Classification of Diseases Tenth Revision; MC: Molluscum Contagiosum; w/v: Weight/Volume.
Introduction
Molluscum Contagiosum (MC), a viral skin infection caused by Molluscipoxvirus, primarily affects children. MC lesions may appear anywhere on the body as round, slightly umbilicated flesh-colored, dome-shaped bumps [1]. The virus is isolated to the human epidermis, but can be transmitted by skin-to-skin contact with infected persons or contaminated objects. Scratching lesions leads to spread to other areas of the body through auto-inoculation [1].
Pediatricians are often the first to recognize and diagnose MC, typically by medical history and physical examination and without the need for laboratory testing or biopsy. The gold standard of MC treatment is a “wait and see” approach, with an expectation of spontaneous lesion clearance [2]. However, some MC infections last months or years [3]. Treatment may be recommended for long term MC cases, young patients, those with several lesions or lesions in sensitive body areas, or for those with severe itching, redness, secondary infection, and/or risk of scarring or spreading to siblings [2,4]. Prior to 2023, there were no FDA approved treatments indicated for MC. Today there are two, one is a drug-device combination of 0.7% w/v cantharidin (Y-CANTH™, Verrica Inc. West Chester, PA) [5] and the other is berdazimer topical gel, 10.3% (Zelsuvmi™, LNHC Inc, Durham, NC), a novel first-in-class topical nitric oxide releasing medication that is anticipated to be available by prescription in the near future [6,7]. The cantharidin drug-device must be applied to MC lesions in a healthcare provider’s office and washed off post application as directed [8]; whereas berdazimer topical gel can be applied to lesions once a day by the patient or caregiver/parent at home and does not require post-treatment wash off [6,9].
Historically, dermatologists and pediatricians have treated MC infections using physical destruction procedures such as curettage or cryotherapy, which must be administered by a healthcare provider within the office setting. Chemical agents including compounded non-prescription cantharidin, and off-label tretinoin, 25% - 50% trichloroacetic acid, silver nitrate, potassium hydroxide, and imiquimod have been used to trigger localized inflammation in an effort to accelerate MC lesion clearance [2,4]. Cryotherapy, curettage, and cantharidin require multiple in-office visits and have potential adverse outcomes, like pain, burning, blistering, localized edema and erythema and discomfort after treatment [2,4].
Natural or treatment-induced MC resolution is often characterized by the development of tender, crusted, erythematous plaques that surround one or more lesions which can be mistaken for treatment side effects and/or secondary infections [10,11]. However, localized inflammation often represents a host response of imminent MC resolution referred to as Beginning-of-the-End (BOTE) sign [11]. Parental anxiety is often cited as a primary reason for treating molluscum and the redness, inflammation and irritation associated with MC treatments and/or the normal healing process may further spur concerns about localized infection [12]. Hence, practitioners may prescribe medications, such as antibiotics, topical steroids or other medications to relieve symptoms [13].
This exploratory analysis sought to determine the claims-based MC incidence in the United States pediatric population and to discern MC management and treatment patterns of pediatricians.
Methods
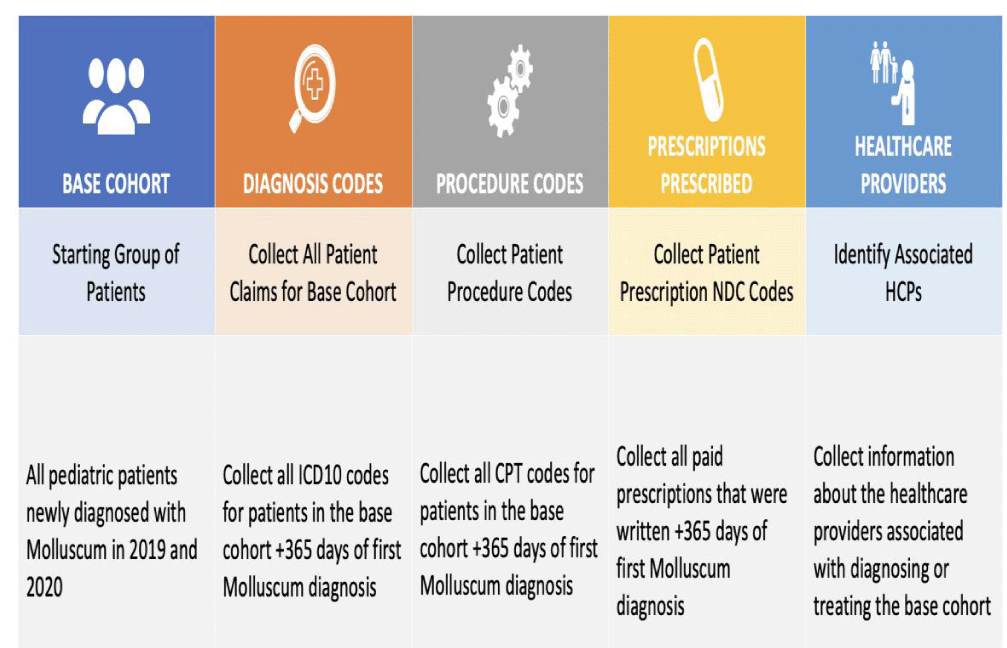
Syneos Health Consulting Insight Suites used the Compile™ Claims database (McKesson, Irving, TX.) to collect all medical and pharmacy claims for pediatric patients newly diagnosed with Molluscum (ICD10 Code B081) in 2019 and 2020. All medical and pharmacy claims for these patients in the 365 days after their first Molluscum diagnosis were captured. (Figure 1).
Next, the number of pediatric patients who had a Molluscum (B081) claim with a pediatrician (pediatrician included nurse practitioners and physician assistants associated with a pediatric practice or having a pediatric specialization) was calculated as well as the number of patients who were prescribed a prescription by the same pediatrician within 30 days of the Molluscum claim. Only paid pharmacy claims were included in this analysis. Compile™ is one of the largest comprehensive datasets of real world closed claims that allows for specific insights into the association between diagnostic codes, patient visits and prescriptions filled. To enhance specificity and sensitivity, only the ICD10 code specific to Molluscum was used in this analysis.
Pharmacy claims typically do not specify for what condition the medication(s) prescribed, therefore we anchored to the medical claims, which do contain diagnosis information, and used a 30-day period to identify prescriptions likely being used off label to treat molluscum.
Pediatricians and dermatologists were segmented into three categories based on their MC diagnosis and management strategies: “wait and see” was defined as those who routinely provided no treatment for the majority of patients within 30 days of MC diagnosis; a “treater” included providers who routinely initiated treatment with either a physical procedure (cryosurgery, cantharidin or curettage) or off label prescription medication, within 30 days of MC diagnosis; and a “referrer” included those who routinely referred patients to another healthcare provider within 30 days of MC diagnosis and did not routinely treat or manage MC. Only frequency and descriptive statistics were captured.
Results
Diagnosis
From January 1, 2019- December 31, 2020, claims analysis identified 260,788 molluscum patients under the age of 19 years diagnosed by 56,026 healthcare providers. Of these healthcare providers, nearly two-thirds were pediatricians (63%, n = 35,017) and 12% (n = 6,553) were dermatologists.
Of the 260,788 MC patients with MC associated claims, most, 72% (n = 187,228), were diagnosed by pediatricians; 18% of patients (48,238) were diagnosed by dermatologists and the remaining 25,322 patients diagnosed by 14,456 (10%) identified as “other.”
Pediatricians were responsible for a majority of molluscum diagnoses, most were affiliated with Integrated Delivery Network (IDN) and provider groups. The majority of MC diagnosing dermatologists were in private practice.
MC management approach
Ninety-one percent (n = 31,968) of pediatricians employed a “wait and see” approach to managing MC, refraining from administering any therapy or referring patients; in contrast 35% (n = 2,307) of dermatologists took this approach. Eight percent (n = 2,783) of pediatricians were treaters (initiating treatment at diagnosis with most MC patients) and 0.8% (n = 266) referred their MC patients to another provider, typically a dermatologist. About 2/3 (65%, n = 4,231) of dermatologists were classified as treaters and only 0.2% (n = 15) routinely referred. Further analysis revealed most patients remain untreated if they are not treated within the first 30 days after MC diagnosis. For practitioners who decided to treat MC, “wait and see” pediatricians treated only about 7% (n = 12,292/175,593) of MC patients whereas treaters treated 73% of MC patients (n = 8,164/11,184).
Treatment preferences
Treaters from both specialties favored procedures over prescriptions: 58% (n = 1,607/2,783) of pediatricians and 94% (n = 3,982/4,231) of dermatologists. If a “wait and see” pediatrician decided to initiate treatment, about half of their MC patients received prescription therapy (49.9%, n = 6,134/12,292), with 52% (n = 6,392/12,292) managed by a procedure; 258 patients received both modalities. Pediatrician treaters managed 81% (n = 6,612/8,164) of their MC patients by procedure and only 23% (n = 1,878/8,164) by prescription.
Among pediatricians who treated MC, the highest rates of treatment were among those residing in the Northeast and Southern regions of the US, with 477 and 423 cases per 100,000 respectively, which was about 110 more MC cases on average per 100,000 pediatric population and a 28% increase over the average National rate versus the Northwest (n = 350) and Midwest (n = 331).
Most pediatricians, 63.5%, (n = 22,228/35,017) diagnosed between 1-20 molluscum patients but a small number, 0.05% treated over 100 patients per year (18/35,017). The pediatrician’s treatment behavior did not vary with the volume of MC patients seen.
Prescription treatment
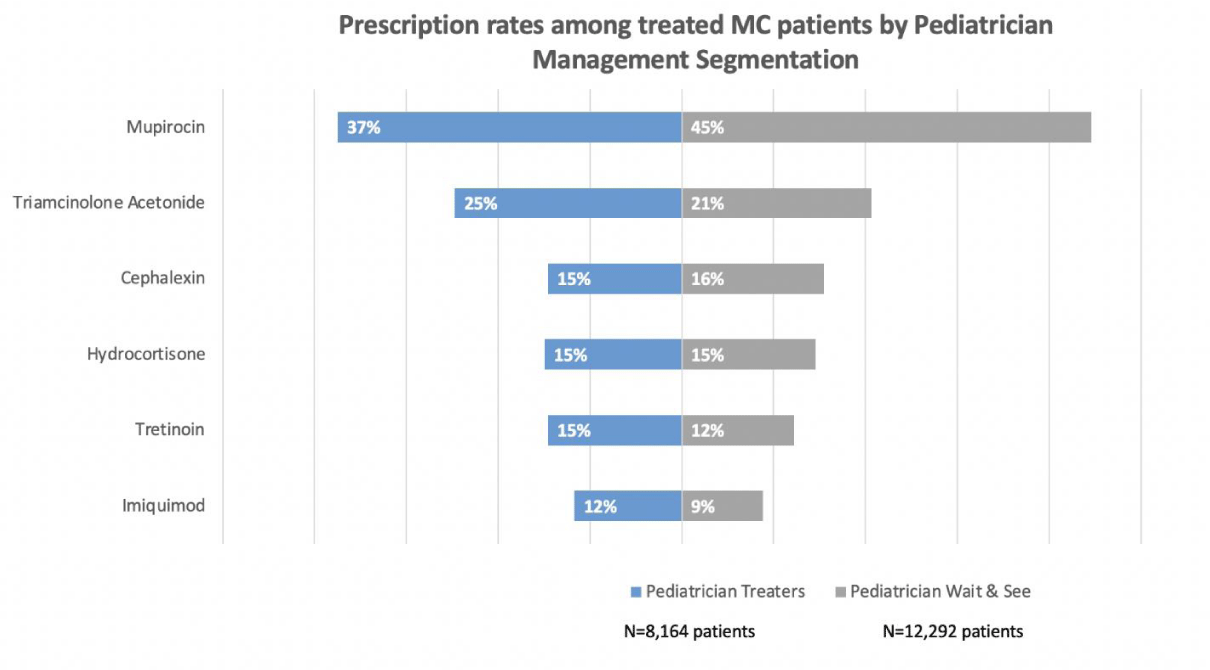
Mupirocin and triamcinolone were the most preferred prescription therapies when pediatricians prescribed medications, regardless of if they were typically a “wait and see” manager or a routine MC treater (Figure 2).
Prescriptions for MC were used at higher rates among IDN/Provider Network pediatrician and those located in the Southern US.
Discussion
This analysis revealed nearly a quarter of a million pediatric patients are diagnosed with MC in the US annually. Most pediatricians take a wait and see approach, whereas most dermatologists treat within 30 days of diagnosis. This finding is consistent with recommendations outlined in the Red Book regarding MC management [2]. Additionally, this could be due to the lack of FDA approved medications at the time of this analysis and the time, tools and skill needed to apply in-office MC procedures, such as cryosurgery, cantharidin application and/or curettage often used by dermatologists [4,13]. When routine treaters initiated therapy, they tended to use procedures whereas wait and see managers favored off-label prescription therapy. Referred patients were generally treated with a procedure. This analysis did not capture the influence of the patient’s age, medical history, lesion location, lesion number or other characteristics on the type of treatment selected.
Pediatricians most frequently prescribed mupirocin and triamcinolone in association with a MC claim; neither drug is FDA approved for MC. MC treatment side effects, as well as MC-disease associated swelling, redness and BOTE mimic cutaneous infection, suggesting these symptoms may be the target and motivation of such treatments. Yet, careful consideration of the cause of these symptoms is warranted in light of increasing concerns about mupirocin resistance [14]. Corticosteroids may be indicated for pruritic molluscum dermatitis but are not suitable to treat BOTE sign [13,15]. The availability of two FDA approved MC treatments will likely change pediatrician’s MC management behaviors.
Conclusion
This study was the first to estimate MC incidence in the pediatric population using recent US claims data. The results demonstrate pediatrician’s adherence to the Red Book’s suggested management practice of “wait and see,” but to consider treatment when warranted. In the absence of MC management consensus guidelines, there continues to be disparate practices suggesting a need for consistent recommendations to guide practice behaviors toward the treatment and resolution of MC in children, particularly with the introduction of two FDA approved treatments indicated for MC.
Limitations
Because there were no FDA approved treatments at the time of this analysis, MC patients may not be diagnostically coded and therefore the claims database may underestimate the actual number of cases. Additionally, claims data were captured in 2019-2020, the latter during COVID closures, and thusly may underestimate the true incidence of MC. The data were extracted from Compile™ using predetermined inputs that precluded analysis of claims by year, therefore claims for the individual years of 2019 versus 2020 were not assessed nor compared. The database does not show what medical condition for which the drug is prescribed. Effectiveness and clinical outcomes of administered MC treatments was not captured.
Conflicts of interest
Drs. Cartwright and Maeda-Chubachi and Ms. Enloe are employees of Pelthos (previously Novan, Inc.). Dr. Stripling is a clinical investigator and advisor for Pelthos, and a clinical investigator for Arcutis Biotherapeutics.
Funding
The claims data collection and manuscript development were funded by Pelthos (previously Novan) Durham, NC, USA. Novan/Pelthos contracted Syneos Health Consulting to collect claims data and collate and analyze the data.
Author contributions
Drs. Cartwright and Maeda-Chubachi conceptualized and designed the study. Dr. Cartwright drafted the initial manuscript. Drs. Maeda-Chubachi and Stripling, and Ms. Enloe critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
- Chen X, Anstey AV, Bugert JJ. Molluscum contagiosum virus infection. Lancet Infect Dis. 2013 Oct;13(10):877-88. doi: 10.1016/S1473-3099(13)70109-9. Epub 2013 Aug 21. PMID: 23972567.
- Committee on Infectious Diseases. American Academy of Pediatrics. Red Book: 2021-2024. Report of the Committee on Infectious Diseases. 32nd ed. American Academy of Pediatrics; 2021.
- American Academy of Dermatology. Molluscum contagiosum: an overview. Accessed December 16, 2023. https://www.aad.org/public/diseases/a-z/molluscum-contagiosum-overview
- van der Wouden JC, van der Sande R, Kruithof EJ, Sollie A, van Suijlekom-Smit LW, Koning S. Interventions for cutaneous molluscum contagiosum. Cochrane Database Syst Rev. 2017 May 17;5(5):CD004767. doi: 10.1002/14651858.CD004767.pub4. PMID: 28513067; PMCID: PMC6481355.
- Verrica Pharmaceuticals Inc. YCANTH™ (cantharidin) topical solution. 2023. https://ycanth.com/wp-content/uploads/2023/07/USPI_FPI-0003_YCANTH.pdf.
- LNHC Inc. ZELSUVMI™ (berdazimer) topical gel [prescribing information]. January 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/217424s000lbl.pdf
- US Food and Drug Administration. Novel drug approvals for 2024. https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2024
- Eichenfield LF, McFalda W, Brabec B, Siegfried E, Kwong P, McBride M, Rieger J, Willson C, Davidson M, Burnett P. Safety and Efficacy of VP-102, a Proprietary, Drug-Device Combination Product Containing Cantharidin, 0.7% (w/v), in Children and Adults With Molluscum Contagiosum: Two Phase 3 Randomized Clinical Trials. JAMA Dermatol. 2020 Dec 1;156(12):1315-1323. doi: 10.1001/jamadermatol.2020.3238. PMID: 32965495; PMCID: PMC7512131.
- Browning JC, Enloe C, Cartwright M, Hebert A, Paller AS, Hebert D, Kowalewski EK, Maeda-Chubachi T. Efficacy and Safety of Topical Nitric Oxide-Releasing Berdazimer Gel in Patients With Molluscum Contagiosum: A Phase 3 Randomized Clinical Trial. JAMA Dermatol. 2022 Aug 1;158(8):871-878. doi: 10.1001/jamadermatol.2022.2721. PMID: 35830173; PMCID: PMC9280611.
- Butala N, Siegfried E, Weissler A. Molluscum BOTE sign: a predictor of imminent resolution. Pediatrics. 2013 May;131(5):e1650-3. doi: 10.1542/peds.2012-2933. Epub 2013 Apr 1. PMID: 23545377.
- Maeda-Chubachi T, Hebert D, Messersmith E, Siegfried EC. SB206, a Nitric Oxide-Releasing Topical Medication, Induces the Beginning of the End Sign and Molluscum Clearance. JID Innov. 2021 May 5;1(3):100019. doi: 10.1016/j.xjidi.2021.100019. PMID: 34909721; PMCID: PMC8659381.
- Olsen JR, Gallacher J, Piguet V, Francis NA. Epidemiology of molluscum contagiosum in children: a systematic review. Fam Pract. 2014 Apr;31(2):130-6. doi: 10.1093/fampra/cmt075. Epub 2013 Dec 2. PMID: 24297468.
- Hebert AA, Bhatia N, Del Rosso JQ. Molluscum Contagiosum: Epidemiology, Considerations, Treatment Options, and Therapeutic Gaps. J Clin Aesthet Dermatol. 2023 Aug;16(8 Suppl 1):S4-S11. PMID: 37636018; PMCID: PMC10453394.
- Patel JB, Gorwitz RJ, Jernigan JA. Mupirocin resistance. Clin Infect Dis. 2009 Sep 15;49(6):935-41. doi: 10.1086/605495. PMID: 19673644.
- Berger EM, Orlow SJ, Patel RR, Schaffer JV. Experience with molluscum contagiosum and associated inflammatory reactions in a pediatric dermatology practice: the bump that rashes. Arch Dermatol. 2012 Nov;148(11):1257-64. doi: 10.1001/archdermatol.2012.2414. PMID: 22911012.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
