Open Journal of Pediatrics and Child Health
Intrapulmonary percussive ventilation for children with bronchiolitis on non-Invasive Ventilation support
Yuval Cavari1*, Tal Levy Shlomo2, Eitan Neeman1, Ben Taragin3, Michal Leder1, Shaked Yarza4 and Isaac Lazar1
2Pediatric Physiotherapy Unit, Soroka University Medical Center affiliated to Ben-Gurion University of the Negev, Israel
3Pediatric Radiology, Medical School for International Health, Israel
4Clinical Research Center, Soroka University Medical Center, Beer Sheva, Israel
Cite this as
Cavari Y, Shlomo TL, Neeman E, Taragin B, Leder M, et al. (2022) Intrapulmonary percussive ventilation for children with bronchiolitis on non-Invasive Ventilation support. Open J Pediatr Child Health 7(1): 025-030. DOI: 10.17352/ojpch.000042Copyright
© 2022 Cavari Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Objective: Pediatric Intensive Care (PICU) admission of children with bronchiolitis as well as the use of Non-Invasive Ventilation (NIV) are increasing. The current treatment for bronchiolitis is supportive, and there are no specific studies addressing this group of severe bronchiolitis patients supported with NIV. Intrapulmonary Percussive Ventilation (IPV) is a lung recruitment physical therapy technique used in our PICU to augment lung aeration and improve gas exchange. We hypothesized that IPV treatment can be used to improve the clinical course of infants on NIV support suffering from bronchiolitis.
Design: A prospective, open, randomized study.
Setting: Single-center Pediatric ICU
Patients: Children less than 2 years old admitted to our PICU between November 2016 and April 2018 with a diagnosis of bronchiolitis who were prescribed noninvasive positive pressure ventilation as their sole respiratory treatment modality
Interventions: Patients were randomly assigned to two intervention groups: IPV vs. control (standard treatment).
Measurements and main results: Thirty-eight infants with bronchiolitis treated with NIV support were randomized into two groups. The probability of a superior outcome (less chance of invasive mechanical ventilation and fewer PICU days) was 62.7% (95% CI, 45%-77%, p = 0.18) in the IPV group compared to the control group. Among the IPV group, there were no failures that required intubation in comparison to three intubations (13.6%) among the control group (p = 0.24). For the IPV group, the PICU length of stay (LOS) was 4.13 ± 2.45 days, compared to 6.18 ± 4.72 for the inhalation group. This difference was not statistically significant.
Conclusions: In this single-center study, the use of IPV had no adverse reactions. The study failed to show a statistically significant effect of IPV treatment on the course of hospitalization of patients with bronchiolitis on NIV support in the PICU.
Trial registration: Clinical Trials.gov NCT03037801.
Introduction
Most children hospitalized due to acute bronchiolitis are managed in the pediatric ward with supportive care: oxygen supplementation, nebulized hypertonic saline, and occasionally intravenous fluids or nasogastric tube feed as tolerated; however, PICU admission rate of infants with acute bronchiolitis is increasing as well as the use of non-invasive ventilation (NIV) [1-3].
Previous studies of severe bronchiolitis focused on predictors of PICU admission, warning signs for deterioration, and risk factors for a more severe course of the disease [4-7]. Younger age, prematurity, and co-morbidity were identified as risk factors for PICU admission [2,7].
The clinical management of acute bronchiolitis remains challenging. In the past decade, pharmacological interventions and chest physiotherapy have failed to show any benefit, making supportive care the hallmark of current therapy [8]. The use of nebulized hypertonic saline has some evidence and is being used in our hospital [9,10]. Despite clinical guidelines, there are major variations between Hospitals and physicians in treating bronchiolitis patients [11].
Intrapulmonary percussive ventilation (IPV) is used for physiotherapy. The IPV delivers small bursts of high-flow gas within a frequency range of 100 – 450 cycles/min. IPV provides a convective front of gas to the distal airways and a more homogenous distribution of alveolar ventilation. IPV promotes alveolar recruitment, helps to “unstick” mucus in small and middle-sized airways, and propels secretions cephalad to the central airways by its asymmetrical flow pattern, whereby expiratory flow exceeds inspiratory flow [12]. IPV improves airway secretion clearance in children with atelectasis [13] and recently has shown a beneficial effect in mild to moderate acute bronchiolitis patients admitted to the pediatric ward [14].
The primary objective of this open randomized clinical trial was to evaluate the effectiveness of IPV in reducing NIV failure and resulting intubation/ conversion to invasive ventilation, in PICU patients admitted with severe bronchiolitis.
To our knowledge, there have been no trials of IPV in children receiving NIV for bronchiolitis.
Materials and methods
Study design
Children under 2 years of age, admitted to the PICU with severe bronchiolitis requiring ventilatory support were recruited during two consecutive bronchiolitis seasons from November 2016 through April 2018. The study was approved by the local institutional review board (IRB) committee. The trial was preregistered with Clinical Trials.gov NCT03037801.Informed consent in Hebrew or Arabic was obtained from one of the parents. The study was performed in an 8-bed PICU of a university-affiliated tertiary care hospital. The trial was preregistered with Clinical Trials.gov€ NCT03037801.
Bronchiolitis was diagnosed clinically (respiratory distress, rales, crackles on chest auscultation, accessory muscle breathing without congenital heart disease) and the decision to support children with NIV was at the discretion of the attending physician. Exclusion criteria were: children 2 years of age or older, patients who were intubated upon arrival, and patients who needed ventilatory support due to underlying organ failure (neurologic or congestive heart failure) other than bronchiolitis. All children had a nasopharyngeal aspirate collection for virology polymerase chain reaction (PCR) testing. Once parental consent was obtained, children were randomized to the different treatment modalities according to admission number, even vs. odd numbers, to which the recruiting physician or nurse was blinded until inclusion.
Ventilatory support was provided by nasal Intermittent Positive Pressure Ventilation (nIPPV) mode via the Leoni Plus ventilator (Löwenstein Medical GmbH & Co. Germany) or High Flow Nasal Cannula (HFNC) (Vapotherm, Exeter, New Hampshire, United States) per our PICU standard policy.
Study intervention
Children were randomly assigned into two arms: IPV and control (standard treatment). Both groups received conventional supportive treatment of intravenous fluids and nasogastric feeds as tolerated by the patient as well as hypertonic saline and salbutamol inhalations per hospital protocol. Salbutamol was added to nebulized hypertonic saline to minimize bronchospasm. During the administration of the inhalation, via a well-fitted mask, the patient was taken off the ventilator. The study protocol did not limit physicians’ perceptional use of steroids or antibiotics. None of the patients received sedation.
The control group received twice daily inhalations of 3ml hypertonic saline (NaCl 3%) with 2.5 mg salbutamol, nebulized over 10 minutes via oxygen flow at 5 L/min (standard protocol in our PICU).
The intervention group received twice daily IPV treatments, 12 ml NaCl 3% with 2.5 mg salbutamol, over 20 minutes, to allow continuous aerosol delivery during treatment. IPV was delivered using a well-fitted mask. We used an Ambu face mask that covers the nose and mouth.
IPV treatments were delivered by specialized pediatric respiratory physiotherapists, experienced in using the IPV. We used IPV 2C device according to our PICU standard protocol at the onset of treatment: Operational pressure of 30 PSI, “Inspiratory time” and “Inspiratory flow” at mid position, CPAP at the lowest pressure (knob to the right) and frequency to the maximum (knob to the left). During the treatment, the physiotherapist increased the flow and decreased the frequency according to tactile feedback from the patient’s chest “wiggling sensation”. Each treatment continued for 20 minutes and at the end of the treatment oral and nasal suction was performed. The study continued as long as the patient was on ventilation support.
Study variables
The primary outcome was the failure of NIV and the need for invasive ventilation.
Secondary Outcomes included: Short-term effect of IPV treatment was measured by Modified Tal Score (MTS), Oxygen saturation/Fraction of inspired oxygen ratio (S/F ratio) and Trans cutaneous Carbon dioxide level (TC CO2). The long-term effect was measured by the length of PICU Stay (LOS) and Lung atelectasis score.
MTS is a clinical severity scoring system that assigns a value between 0 and 3 to each of four variables: respiratory rate, wheezing/crackles, oxygen saturation (at room air), and the use of accessory muscles. All subjects in our study received a score of 3 for oxygen saturation as they all required supplemental oxygen. Higher MTS indicates increased severity of bronchiolitis. MTS has strong validity and reliability and correlates with outcomes [15].
Fractional oxygen (FiO2) on the NIV support (nIPPV or HFNC) was adjusted to the lowest level which targeted oxygen saturation (SaO2) higher than 93%. This was done to enable the calculation of the S/F ratio.
TC CO2 (Sen Tec AG, Switzerland) measurements were added to all subjects during the second consecutive year. Before treatment intervention (hypertonic saline inhalation or IPV) the patient was connected to TC CO2 and the intervention began after receiving a steady signal reading.
These parameters (MTS, S/F ratio, and TC CO2) were obtained for each patient before the intervention, 15 minutes, and 30 minutes post-intervention. Practically every patient in the study had a datasheet beside the bed where the patients’ nurse recorded in real-time, as the vital signs stabilized, the respiratory rate, wheezing/crackles on auscultation, use of accessory muscles, SaO2, FiO2 and TC CO2. Once the baseline measurements were recorded the intervention was immediately commenced.
Chest radiographs were obtained every other day and reviewed retrospectively by a pediatric radiologist blinded to the type of treatment the patient received. Chest X-rays were graded according to atelectasis score –a value between 0 and 4 was assigned, corresponding to a complete resolution of collapse to complete collapse of ≥ 2 segments or lobes [13,16].
Statistical analysis
Descriptive statistics: Demographic and clinical characteristics were compared at baseline. Continuous variables with normal distribution are presented with mean and standard deviation (SD) and were compared using a parametric T-Test. Categorical variables are presented as a count percent of the total and compared using Pearson’s χ2 test for contingency tables or Fisher Exact test, as appropriate. Analyses were performed using SPSS (Version 22, Chicago, IL) and R statistical software version 3.5.1.
Linear mixed model: To estimate the effect of IPV treatment on the change in TC CO2 measurements during the treatments a linear mixed model was utilized, in which individuals were included as a random effect. This was done to control for the cluster effect of the repeated treatments of the same patient. TC CO2 measurements in time 0 and the number of treatments were included as a fixed effect.
Hierarchical composite endpoint: Finkelstein and Schoenfeld methods [17] were used to calculate the hierarchical composite endpoint. In this analysis, each patient was compared with every other patient in the trial, both cases, and controls. In every comparison, a score of +1 is given for the patient who had a better outcome compared to the other subject, a score of -1 if he had a worse outcome and a score of 0 for the same outcome. The ranked composite outcome score incorporated the necessity for invasive mechanical ventilation, which is the first prominent outcome, and PICU days as the secondary outcome measure. If one patient needed invasive mechanical ventilation and the other did not, scores of -1 and +1 were set, respectively. In a case where both subjects required invasive mechanical ventilation, both received a score of 0. If neither of the compared subjects required invasive mechanical ventilation, the score was set based on the PICU days: A patient with a lower number of PICU days was set a score of +1 while one with a higher number of PICU days was set a score of -1. Similar PICU days duration set a score of 0 for both subjects. The pairwise comparisons were all summed for each patient, who received a rank based on his cumulative score. These ranks are compared using the Mann-Whitney test.
The effect size is reported as the probability of superiority, also known as the probabilistic index, which describes the probability for a randomly selected subject to have a better outcome than a different randomly selected subject from the other group [18]; 95% CI were calculated as described by Newcombe [19].
Results
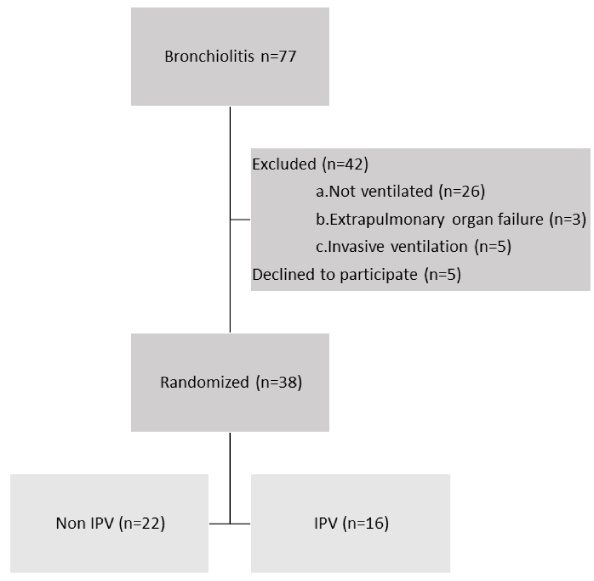
Thirty-eight infants which met inclusion and exclusion criteria were randomized: 22 in the control group and 16 in the IPV group as depicted in Figure 1. 32 (84%) patients were supported on nIPPV and 6 (3 in each group) patients were supported on HFNC 2 Liters/Kg. No patient dropped off the study once recruited.
Patient demographics and clinical severity data are reported in Tables 1,2 respectively. The infants in the IPV group were younger, weighed less, and included more ex-preterm infants. The prominent virus in this study was the Respiratory Syncytial virus (RSV), other viruses in both groups included Rhinovirus, Enterovirus, and Parainfluenza virus. Baseline severity score expressed as MTS, S/F ratio, and chest x-ray score was comparable between groups, but the IPV group had higher TC CO2 at baseline.
Primary outcome measure
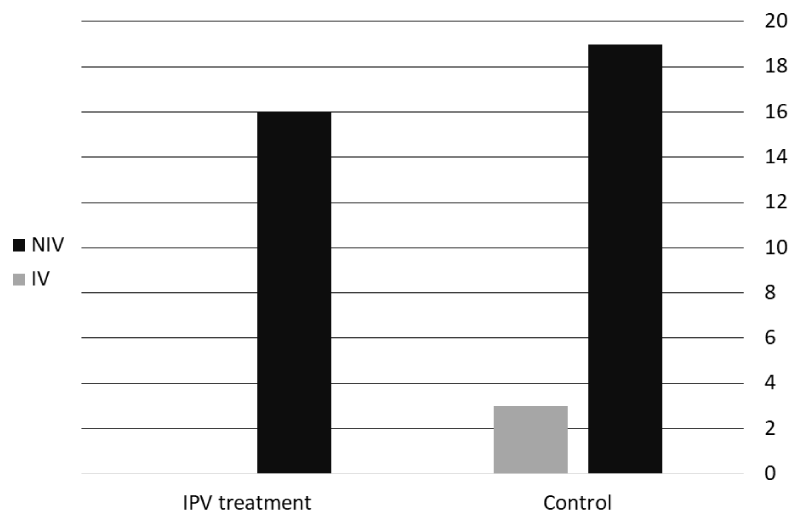
None of the subjects in the IPV group required invasive ventilation, compared to three in the control group (13.6%; p = 0.24), as seen in Figure 2. The probability of a superior outcome (less chance of invasive mechanical ventilation and fewer PICU days) was 62.7% (95% CI, 45%-77%, p = 0.18) in the IPV group compared to the control group.
Secondary outcome
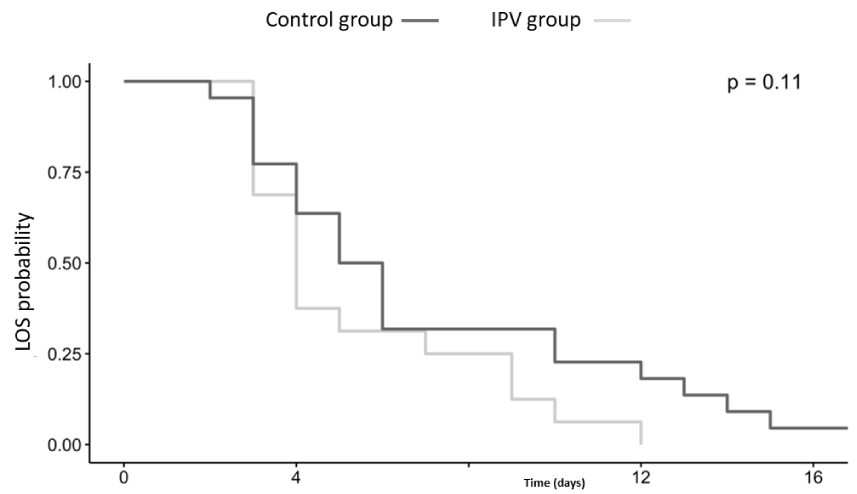
Overall, no significant difference in the PICU LOS between groups was found (p = 0.11). For the IPV group, PICU days were 4.13 ± 2.45 in comparison to 6.18 ± 4.72 for the control group. Figure 3 shows the Kaplan-Meier plot for the two populations. In addition, we found no significant association between treatment and effect in short-term variables of clinical severity score evaluated in MTS, oxygenation expressed as S/F ratio, and ventilation evaluated by TC CO2 (Table 3). In the IPV group, there was a decrease in TC CO2 (coefficient= -1.12, 95% CI, -4.28; 2.13) but MTS has increased, (coefficient= 0.22, 95% CI, -0.003; 0.46) at 15 minutes after treatment. This trend of decreased TC CO2 and concomitant increase in MTS was also observed 30 minutes after treatment. Chest x-ray evaluation of lung atelectasis score had no difference between groups (p = 0.55).
Discussion
This randomized controlled trial assessed the effectiveness of IPV treatment in non-invasively ventilated children with severe bronchiolitis in a single-center PICU. The primary outcome was the prevention of intubation and invasive ventilation. The secondary outcome was adverse events of IPV, PICU days, and the effect on oxygenation and ventilation. Due to the high prevalence of this disease and the associated morbidity and mortality, any beneficial intervention which may lower complications or decrease LOS should have a major clinical and economic impact. In our study cohort, none of the children with bronchiolitis treated with IPV had to be intubated and ventilated compared to three in the control group, however, we failed to show statistical significance between the groups. Our failure to meet significance could be secondary to several possible causes: IPV brings no improvement to infants with bronchiolitis or, our study did not have the power to show significant clinical benefit with a methodological bias between the study groups. Our clinical experience supports the latter cause.
In the IPV treatment group, infants were younger, their weight was lower and the prematurity rate was higher. Because all these parameters are known to increase the severity of bronchiolitis [7,8] we believe that any beneficial result cannot be explained by the difference in demographics. Despite the vulnerability of the infants in the intervention group, namely a mean age of 35.8 days, a 50% preterm rate, and a mean weight of 3.4 kilograms, there were no adverse events during or after treatment. While conventional physiotherapy, which includes vibration and percussion, is often not well tolerated in infants with bronchiolitis, our experience and findings show good tolerance to IPV. This may be explained by the theory that IPV provides a convective front of gas to the distal airways, avoiding airway collapse. This intrapulmonary approach, unlike the chest wall approach, does not aggravate chest wall compliance [20]. Another advantage of this technique, which is especially important in infants, is that active cooperation is not necessary to perform the treatment.
Hospitalization of children with bronchiolitis in the PICU is increasing, as is the use of mechanical ventilation for these patients. Ghazaly and Nadel [7] found that the median LOS for children admitted to the PICU with acute bronchiolitis was 6 days. They also identified prematurity and younger age as risk factors for prolonged LOS. In our study, the mean length of stay was 6.18 days for the control group, versus 4.13 days for the IPV group. Van Ginderdeuren, et al. [14] found a significant reduction of 1-day stay between both treated groups: Assisted autogenic drainage or IPV and the control group. Our study’s cohort is different; while Van Ginderdeuren studied patients with mild to moderate bronchiolitis, receiving one treatment a day and admitted to the pediatric ward, we focused on the more severely ill patients who require NIV, are treated twice daily, and reside in the PICU. Since bronchiolitis in most cases is a self-limiting disease, the LOS plays an important role in evaluating the effectiveness of treatment. We speculate that the ability to intervene in the first stages of the disease is probably limited, as we see patients who despite receiving proper NIV support continue to be tachypneic with increased work of breathing. Therefore, the decrease in LOS may be due to the prevention of complications. Recent work by Kepreotes [21] evaluating HFNC for bronchiolitis also concluded that the modality of oxygen support does not modify the underlying disease process but may have a role in reducing the proportion of children requiring intensive care.
During the second year of our study, we added another objective parameter of TC CO2 that has been found to correlate with disease severity [22]. A hallmark characteristic of bronchiolitis is ventilation heterogeneity encompassing areas of poor ventilation due to atelectasis and areas of overdistention. This pathophysiology leads to increased dead space ventilation and a subsequent increase in PaCO2. Atelectasis, on one hand, causes a localized low lung volume state with narrow extra-alveolar vessels. Simultaneously, overdistention secondary to auto-PEEP causes stretching and narrowing of capillaries. IPV provides a more homogenous distribution of alveolar ventilation maintaining airways open and resolution of atelectasis [13,16]. Following this assumption, in our study TC CO2 levels decreased at 15 minutes post-intervention and to a greater extent at 30 minutes post-treatment. However, this decline was not statistically significant.
The major limitations of this study are the low number of infants recruited during the study period and to a greater extent, the interpretation of TC CO2 levels that were collected only during the second year. Another limitation was that besides supportive treatment, the control group received inhalation treatment, which may have had some effect. We also acknowledge the difference in the amount of hypertonic saline in the IPV group (12 ml) compared to the control group (3 ml) this difference lies in the treatment modalities. A further limitation was the use of a non-validated clinical score system for severe bronchiolitis patients on ventilator support. Another limitation is the variability between physiotherapists; despite using trained experienced physiotherapists for IPV, some inevitable variability is difficult to evaluate and standardize. The problem of practitioner variability for complex interventions is common in nonpharmacological trials.
Conclusion
In this small, prospective Single center cohort of severely ill infants with bronchiolitis, no side effects were observed with IPV treatment. While our findings failed to show significant beneficial effects, there is a trend toward a decreased need for invasive ventilation and improved CO2 clearance. Future prospective studies should aim to further evaluate the effect of IPV in this population.
- Hasegawa K, Tsugawa Y, Brown DF, Mansbach JM, Camargo CA Jr. Trends in bronchiolitis hospitalizations in the United States, 2000-2009. Pediatrics. 2013 Jul;132(1):28-36. doi: 10.1542/peds.2012-3877. Epub 2013 Jun 3. PMID: 23733801; PMCID: PMC3691534.
- Schlapbach LJ, Straney L, Gelbart B, Alexander J, Franklin D, Beca J, Whitty JA, Ganu S, Wilkins B, Slater A, Croston E, Erickson S, Schibler A; Australian & New Zealand Intensive Care Society (ANZICS) Centre for Outcomes & Resource Evaluation (CORE) and the Australian & New Zealand Intensive Care Society (ANZICS) Paediatric Study Group. Burden of disease and change in practice in critically ill infants with bronchiolitis. Eur Respir J. 2017 Jun 1;49(6):1601648. doi: 10.1183/13993003.01648-2016. PMID: 28572120.
- Essouri S, Baudin F, Chevret L, Vincent M, Emeriaud G, Jouvet P. Variability of Care in Infants with Severe Bronchiolitis: Less-Invasive Respiratory Management Leads to Similar Outcomes. J Pediatr. 2017 Sep;188:156-162.e1. doi: 10.1016/j.jpeds.2017.05.033. PMID: 28602381.
- Damore D, Mansbach JM, Clark S, Ramundo M, Camargo CA Jr. Prospective multicenter bronchiolitis study: predicting intensive care unit admissions. Acad Emerg Med. 2008 Oct;15(10):887-94. doi: 10.1111/j.1553-2712.2008.00245.x. Epub 2008 Sep 15. PMID: 18795902.
- Corneli HM, Zorc JJ, Holubkov R, Bregstein JS, Brown KM, Mahajan P, Kuppermann N; Bronchiolitis Study Group for the Pediatric Emergency Care Applied Research Network. Bronchiolitis: clinical characteristics associated with hospitalization and length of stay. Pediatr Emerg Care. 2012 Feb;28(2):99-103. doi: 10.1097/PEC.0b013e3182440b9b. PMID: 22270499.
- Freire G, Kuppermann N, Zemek R, et al; Pediatric Emergency Research Networks (PERN). Predicting Escalated Care in Infants With Bronchiolitis. Pediatrics. 2018;142(3):e20174253. Pediatrics. 2019 Feb;143(2):e20183404. doi: 10.1542/peds.2018-3404. Erratum for: Pediatrics. 2018 Sep;142(3): PMID: 30705143.
- Ghazaly M, Nadel S. Characteristics of children admitted to intensive care with acute bronchiolitis. Eur J Pediatr. 2018 Jun;177(6):913-920. doi: 10.1007/s00431-018-3138-6. Epub 2018 Apr 13. PMID: 29654399; PMCID: PMC5958152.
- Florin TA, Plint AC, Zorc JJ. Viral bronchiolitis. Lancet. 2017 Jan 14;389(10065):211-224. doi: 10.1016/S0140-6736(16)30951-5. Epub 2016 Aug 20. PMID: 27549684; PMCID: PMC6765220.
- Zhang L, Mendoza-Sassi RA, Wainwright C, Klassen TP. Nebulised hypertonic saline solution for acute bronchiolitis in infants. Cochrane Database Syst Rev. 2017 Dec 21;12(12):CD006458. doi: 10.1002/14651858.CD006458.pub4. PMID: 29265171; PMCID: PMC6485976.
- Heikkilä P, Renko M, Korppi M. Hypertonic saline inhalations in bronchiolitis-A cumulative meta-analysis. Pediatr Pulmonol. 2018 Feb;53(2):233-242. doi: 10.1002/ppul.23928. Epub 2017 Dec 21. PMID: 29266869.
- Carande EJ, Pollard AJ, Drysdale SB. Management of Respiratory Syncytial Virus Bronchiolitis: 2015 Survey of Members of the European Society for Paediatric Infectious Diseases. Can J Infect Dis Med Microbiol. 2016;2016:9139537. doi: 10.1155/2016/9139537. Epub 2016 Oct 20. PMID: 27840650; PMCID: PMC5093249.
- Fornasa E, Ajčević M, Accardo A. Characterization of the mechanical behavior of intrapulmonary percussive ventilation. Physiol Meas. 2013 Dec;34(12):1583-92. doi: 10.1088/0967-3334/34/12/1583. Epub 2013 Oct 28. PMID: 24165323.
- Deakins K, Chatburn RL. A comparison of intrapulmonary percussive ventilation and conventional chest physiotherapy for the treatment of atelectasis in the pediatric patient. Respir Care. 2002 Oct;47(10):1162-7. PMID: 12354335.
- Van Ginderdeuren F, Vandenplas Y, Deneyer M, Vanlaethem S, Buyl R, Kerckhofs E. Effectiveness of airway clearance techniques in children hospitalized with acute bronchiolitis. Pediatr Pulmonol. 2017 Feb;52(2):225-231. doi: 10.1002/ppul.23495. Epub 2016 Jun 2. PMID: 27254132.
- Golan-Tripto I, Goldbart A, Akel K, Dizitzer Y, Novack V, Tal A. Modified Tal Score: Validated score for prediction of bronchiolitis severity. Pediatr Pulmonol. 2018 Jun;53(6):796-801. doi: 10.1002/ppul.24007. Epub 2018 Apr 14. PMID: 29655288.
- Yen Ha TK, Bui TD, Tran AT, Badin P, Toussaint M, Nguyen AT. Atelectatic children treated with intrapulmonary percussive ventilation via a face mask: clinical trial and literature overview. Pediatr Int. 2007 Aug;49(4):502-7. doi: 10.1111/j.1442-200X.2007.02385.x. PMID: 17587276.
- Finkelstein DM, Schoenfeld DA. Combining mortality and longitudinal measures in clinical trials. Stat Med. 1999 Jun 15;18(11):1341-54. doi: 10.1002/(sici)1097-0258(19990615)18:11<1341::aid-sim129>3.0.co;2-7. PMID: 10399200.
- Acion L, Peterson JJ, Temple S, Arndt S. Probabilistic index: an intuitive non-parametric approach to measuring the size of treatment effects. Stat Med. 2006 Feb 28;25(4):591-602. doi: 10.1002/sim.2256. Erratum in: Stat Med. 2007 Aug 15;26(18):3524. PMID: 16143965.
- Newcombe RG. Confidence intervals for an effect size measure based on the Mann-Whitney statistic. Part 2: asymptotic methods and evaluation. Stat Med. 2006 Feb 28;25(4):559-73. doi: 10.1002/sim.2324. PMID: 16217835.
- Roqué i Figuls M, Giné-Garriga M, Granados Rugeles C, Perrotta C, Vilaró J. Chest physiotherapy for acute bronchiolitis in paediatric patients between 0 and 24 months old. Cochrane Database Syst Rev. 2016 Feb 1;2(2):CD004873. doi: 10.1002/14651858.CD004873.pub5. PMID: 26833493; PMCID: PMC6458017.
- Kepreotes E, Whitehead B, Attia J, Oldmeadow C, Collison A, Searles A, Goddard B, Hilton J, Lee M, Mattes J. High-flow warm humidified oxygen versus standard low-flow nasal cannula oxygen for moderate bronchiolitis (HFWHO RCT): an open, phase 4, randomised controlled trial. Lancet. 2017 Mar 4;389(10072):930-939. doi: 10.1016/S0140-6736(17)30061-2. Epub 2017 Feb 2. PMID: 28161016.
- Gal S, Riskin A, Chistyakov I, Shifman N, Srugo I, Kugelman A. Transcutaneous PCO2 monitoring in infants hospitalized with viral bronchiolitis. Eur J Pediatr. 2015 Mar;174(3):319-24. doi: 10.1007/s00431-014-2407-2. Epub 2014 Aug 28. PMID: 25164063.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
