Journal of Vaccines and Immunology
Evaluation of Anti-SARS-CoV-2 IgG antibody response following COVISHEILD vaccination: A comparison between previously infected and non-infected cohorts
Md Shakeel Ahmed1*, Md Zakir Hossain1, Md Mamunur Rashid1, Istiak Ahmad1, Md Zahirul Islam2, Meherab Hossain1 and Hasan Rabbi1
2Institute for Developing Science and Health Initiatives (ideSHi), Dhaka-1216, Bangladesh
Cite this as
Ahmed MS, Hossain MZ, Rashid MM, Ahmad I, Islam MZ, et al. (2023) Evaluation of Anti-SARS-CoV-2 IgG antibody response following COVISHEILD vaccination: A comparison between previously infected and non-infected cohorts. J Vaccines Immunol 9(1): 030-036. DOI: 10.17352/jvi.000058Copyright
© 2023 Ahmed MS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Background: The Coronavirus disease-2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a significant global health threat. In this study, we investigated the antibody response in five-time intervals following the COVISHEILD first, second, and booster doses vaccination in previously infected and previously non-infected individuals.
Methods: The study was a cross-sectional prospective study that took place at the Bangladesh Institute of Tropical and Infectious Diseases (BITID), Fouzderhat, Chittagong, in 46 individuals who received the COVISHEILD vaccine from February 2021 to January 2022. Blood samples were collected from vaccine recipients at five different time points (Baseline: Day 0 before 1st vaccine dose, 3 weeks, 2 months (before 2nd dose), 6 months, and 1 year after a booster dose) to measure the levels of S-RDB IgG antibodies using the EUROIMMUN Anti-S-Rose Disease Bioinfection assay test kits (Lübeck, Germany).
Results: The study reveals that individuals with prior SARS-CoV-2 infection showed a significant increase in antibody levels after receiving the first vaccine dose, reaching 145.51 units at 3 weeks post-vaccination. This response remained stable at 117.6 units at 3 months and slightly declined to 103.26 units at 6 months, indicating a sustained immune response. For previously non-infected individuals, vaccination induced a strong immune response, with antibody levels of 159.62 units at 3 weeks, increasing to 150 units at 3 months, and then slightly declining to 87.84 units at 6 months. Despite the decline, antibody levels at 6 months and 1 year were notably higher than the pre-vaccination baseline of 0 units, indicating the development of a durable immune response following vaccination. In the <40 years age group, individuals with prior SARS-CoV-2 infection showed a substantial boost in antibody levels after receiving the first vaccine dose, reaching 198.61 units at 3 weeks post-vaccination. The response remained stable at 122.22 units at 3 months and declined to 73.7 units at 6 months, followed by a rise to 263.85 units at 1 year.
Conclusion: Our findings highlight that tailoring vaccination approaches based on gender differences and considering vaccination in both previously and non-infected individuals will aid in optimizing immune responses and combatting the COVID-19 epidemic effectively.
Introduction
The Coronavirus disease-2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a significant global health threat. While many individuals have recovered from mild to moderate respiratory illnesses without specific treatment, elderly individuals with underlying medical conditions such as chronic respiratory disease, diabetes, hypertension, cardiovascular disease, and cancer are more prone to develop severe illness [1]. The COVID-19 pandemic has significantly impacted worldwide, with over 623 million confirmed cases and 6.55 million deaths reported [2,3]. When infected with SARS-CoV-2, the human body produces antibodies targeting the virus spike protein (S) and nucleoprotein (N). Various studies have examined the decline in antibody levels after infection, with some indicating a decrease within four months and others showing sustained presence for up to nine months, albeit at lower levels [4].
The utilization of safe and efficacious vaccines is crucial in preventing the transmission of infections within the population. Vaccination plays a vital role in safeguarding individuals and minimizing the likelihood of severe outcomes in the event of an infection. However, an ongoing concern lies in protecting vaccinated individuals and those who have previously been infected from emerging viral variants, such as the Omicron variant, which are increasingly prevalent among the population [5,6].
Recent research on COVID-19 patients and vaccinated individuals suggests that immunization and vaccination can reduce the occurrence of SARS-CoV-2 infections [7,8]. In line with the recommendations from the World Health Organization (WHO), the government of Bangladesh procured and collected several WHO-recommended COVID-19 vaccines to vaccinate its population. One of the authorized vaccines for emergency use in Bangladesh is COVISHIELD, which is based on the Oxford-AstraZeneca formulation. The Bangladesh Institute of Tropical and Infectious Diseases (BITID) initiated the COVISHIELD vaccination program in February 2021, with the second dose administered in April 2021, following a two-month interval. A third dose was given one year after the second dose. In our study, we investigated the antibody response in five-time intervals following the COVISHEILD first, second, and booster doses vaccination in previously SARS-CoV-2 infected and previously non-infected individuals (so-called naïve individuals).
Methods and Materials
Study design
The study was a cross-sectional prospective study. All the study participants were categorized into two groups: (a) previously non-infected with SARS-CoV-2 virus: No previous COVID-19 history or negative COVID-19 RT-PCR whenever tested, no anti-SARS-CoV-2 antibody (< 8 RU/ml) on the day of first vaccination, and (b) previously infected: Has previous COVID-19 history or positive COVID-19 RT-PCR whenever tested and presence of anti-SARS-CoV-2 antibody ≥ 8 RU/ml on the day of first vaccination. Blood samples were collected from vaccine recipients at five different time points (Baseline: Day “0” before 1st vaccine dose, 3 weeks, 2 months (before 2nd dose), 6 months, and 1 year after a booster dose) to measure the levels of S-RDB IgG antibodies using the EUROIMMUN Anti-SARS-CoV-2 IgG assay test kits (Lübeck, Germany). All of the participants were given the COVISHEILD (ChAdOx1 nCoV-19 mRNA vaccine) vaccine (also known as the Oxford-AstraZeneca vaccine) at least twice, with a minimum interval of 2 months between doses. The vaccine was given as two separate injections, each containing 0.3 mL of the diluted vaccine, which were administered into the deltoid muscle of one of the participant’s upper arms. The initial measurement of antibody levels, or titer, was taken before the first dose of the vaccine (considered as the baseline), and subsequent measurements were taken at 3 weeks, 3 months, 6 months, and 1 year from the day of receiving the 1st vaccine dose.
Study location and Sample Size
The study took place at the Bangladesh Institute of Tropical and Infectious Diseases (BITID), Fouzderhat, Chittagong among 46 individuals who received the COVISHEILD vaccine from February 2021 to January 2022.
Sample selection criteria
The study targeted up to 100 individuals who received the first dose of the COVISHEILD vaccine at the Bangladesh Institute of Tropical and Infectious Diseases (BITID), Fouzderhat, Chittagong from February 2021 to January 2022 covering both male and female patients of different ages. However, participants who were committed to returning to the hospital at five different periods during follow-up and hence were included in the study. However, participants who did not provide written consent were excluded.
Participant’s consent and ethical review
The participants in the study gave written consent after being informed of the study’s purpose, and the research adhered to the ethical standards set by the Declaration of Helsinki. The study only included participants who provided their signatures on a written informed consent form. The participants were then asked to complete a questionnaire that asked for their demographic information, symptoms, and any additional information about comorbidities. The study ensured that no information that could identify individual patients was released or published. Furthermore, the BITID ethical review committee approved the study’s procedures.
Immunological assay
The assay kit was an ELISA test utilizing a plate with wells coated with recombinant Spike (S) protein antigen. The sample testing was performed automatically by the EUROIMMUN analyzer as per the manufacturer’s instructions. Briefly, samples were first diluted 1:101 in the sample buffer provided with the kit. 100 μl each of the calibrator, positive and negative controls, or diluted patient samples were then transferred into the individual microplate wells and incubated for 60 minutes at (37±1) °C. Reagent wells were washed 3 times with 450 μl of working-strength wash buffer. 100 μl of enzyme conjugate was added into each of the microplate wells and incubated for 30 minutes at (37±1) °C. Reagent wells were washed again 3 times with 450 μl of working-strength wash buffer. 100μl of substrate solution was added to each microplate well and incubated for 30 minutes at room temperature (18 °C-25 °C). Finally, 100 μl of stop solution was added into each of the microplate wells and photometric measurement of the color intensity was made at a wavelength of 450 nm and a reference wavelength between 620 nm and 650 nm within 30 minutes of adding the stop solution.
Result interpretation
- <8 RU/ml – negative
- ≥ 8 RU/ml to <11 RU/ml – borderline
- ≥ 11 RU/ml – positive.
Statistical analysis
Data were reported as mean ± standard deviation. Differences between groups were tested with the independent sample t-test. Categorical variables were reported as numbers and percentages and compared between groups using the chi-squared test. The difference in Antibody Responses between repeated measures was tested with the paired sample t-test. A p - value of 0.05 or less was considered statistically significant. All analyses were conducted using Statistical Package for Social Science) for Windows version 23 software.
Results
In individuals with prior SARS-CoV-2 infection (Figure 1A), the immune response to vaccination displayed a notable increase in antibody levels after receiving the first vaccine dose, reaching 145.51 units at 3 weeks post-vaccination. This response remained relatively stable at 117.6 units at 3 months and only slightly declined to 103.26 units at 6 months, yet remained significantly higher than the baseline level of 26.87 units. The antibody level further increased to 224.04 units at 1-year post-vaccination, indicating a sustained immune response in previously infected individuals. For previously non-infected individuals (Figure 1B), vaccination induced a strong immune response, with antibody levels of 159.62 units at 3 weeks, which increased to 150 units at 3 months, and then slightly declined to 87.84 units at 6 months. Despite the decline, the antibody levels at 6 months and 1 year were notably higher than the pre-vaccination baseline of 0 units, indicating the development of a durable immune response following vaccination Table 1.
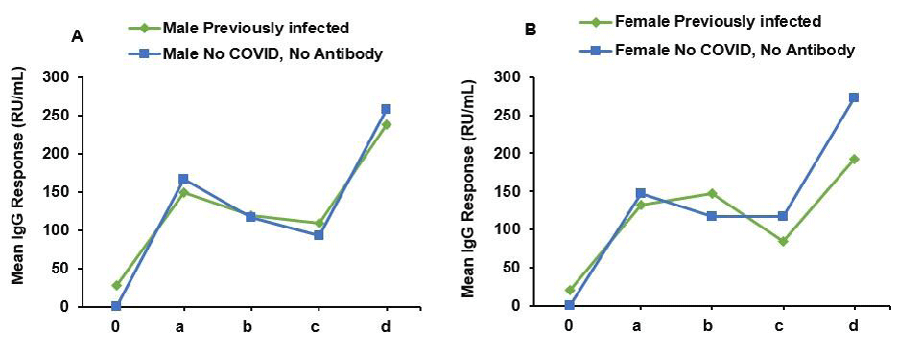
In the male group, individuals with prior SARS-CoV-2 infection displayed an initial antibody level of 28.57 units before vaccination, which significantly increased to 148.73 units at 3 weeks post-vaccination (Figure 2A). The response remained stable at 119.2 units at 3 months and declined slightly to 109.39 units at 6 months, followed by an increase to 237.41 units at 1 year. Males without prior infection exhibited an antibody response to vaccination, with antibody levels of 166.12 units at 3 weeks, which decreased to 117.21 units at 3 months, and then slightly declined further to 93.48 units at 6 months, followed by a rise to 258.1 units at 1 year. Among females, those with prior infection had an initial antibody level of 20.08 units before vaccination, reaching 132.65 units at 3 weeks, and subsequently increasing to 147.57 units at 3 months (Figure 2B). The levels then declined to 84.18 units at 6 months and rose again to 192.46 units at 1 year. Females without prior infection displayed an antibody response to vaccination, with antibody levels of 147.56 units at 3 weeks, slightly decreasing to 117.18 units at 3 months, and remaining stable at 116.84 units at 6 months, followed by an increase to 273.06 units at 1 year.
In the < 40 years age group, individuals with prior SARS-CoV-2 infection showed a substantial boost in antibody levels after receiving the first vaccine dose, reaching 198.61 units at 3 weeks post-vaccination. This response remained steady at 156.38 units at 3 months and only slightly declined to 155.53 units at 6 months, still significantly higher than the baseline. The antibody level further increased to 230.68 units 1 year after the booster dose, indicating a lasting immune response. In contrast, previously non-infected individuals in this age group exhibited a strong response to vaccination, with antibody levels of 160 units at 3 weeks, increasing to 179.73 units at 3 months and 201.28 units at 1 year but overall antibody response was comparatively lower than the previously infected individuals (Figure 3A). For the 40 to <60 years age group, previously infected individuals displayed an initial antibody level of 19.28 units before vaccination, which significantly increased to 135.41 units at 3 weeks post-vaccination. The response remained stable at 122.22 units at 3 months and declined to 73.7 units at 6 months, followed by a rise to 263.85 units at 1 year. Previously non-infected individuals in this age category exhibited an impressive response to vaccination, with antibody levels of 165.9 units at 3 weeks, increasing to 167.04 units at 3 months, and further rising to 277.74 units at 1 year (Figure 3B) but overall antibody response was slightly higher than the previously infected individual. For participants aged ≥ 60 years, those with prior infection started with an antibody level of 21.32 units before vaccination, peaking at 126.97 units at 3 weeks, and subsequently declining to 87.93 units at 3 months. The levels remained relatively stable at 91.6 units at 6 months and 181.18 units at 1 year. Previously non-infected individuals in this age group exhibited a notable response to vaccination, with antibody levels of 142.71 units at 3 weeks, decreasing slightly to 117.18 units at 3 months, and then stabilizing at 116.84 units at 6 months and 258.81 units at 1 year (Figure 3C). Overall antibody response was slightly higher than the previously infected individual.
Discussion
The research study provides valuable insights into the immune responses to SARS-CoV-2 infection and vaccination, taking gender and age groups into account. Our findings reveal that both prior infection and vaccination play crucial roles in eliciting strong and long-lasting immune responses in both males and females. Notably, previously infected individuals from both genders exhibited an initial immune response that was further enhanced by vaccination, resulting in sustained levels of antibodies over the one-year follow-up period. This underscores the importance of vaccinating individuals with a history of infection to bolster their immune response and ensure lasting protection against future infections.
The humoral immune response against SARS-CoV-2 in individuals with a previous history of SARS-CoV-2 infection and non-infected participants has been discussed in several studies [9-12]. Moreover, the antibody response of different COVID-19 vaccines was assessed in males, females, and different age groups [13-16].
Moreover, we observed some variation in immune responses between males and females, with females generally showing higher initial antibody levels before vaccination. However, both genders demonstrated comparable antibody levels at various post-vaccination time points, indicating consistent efficacy of vaccination in inducing protective immunity across sexes. Additionally, the dynamics of immune responses displayed fluctuations over time, possibly influenced by factors such as age, overall health, or genetic differences.
The results of this study underscore the significance of vaccination as a vital tool in generating protective immunity for both genders, irrespective of previous infection status [17-19]. Tailoring vaccination strategies to consider gender differences may be beneficial in optimizing vaccine-induced immune responses, especially in previously non-infected individuals. These findings contribute to our understanding of the immune response to SARS-CoV-2 infection and vaccination, providing valuable insights for informing public health policies and vaccination campaigns aimed at mitigating the impact of COVID-19 in diverse populations [20-23].
Furthermore, the research study sheds light on the immune response following SARS-CoV-2 infection and vaccination across various age groups. Overall, our findings highlight the importance of both prior infection and vaccination in developing an enduring immune response. Among individuals with prior infection, there was a strong initial immune response, which was further augmented by vaccination, resulting in sustained antibody levels over time. Similarly, individuals without previous infection also demonstrated substantial immune responses following vaccination, emphasizing the importance of vaccination in establishing protective immunity, especially in those without prior exposure to the virus [24,25].
Interestingly, the immune response exhibited some differences across age groups, with the younger cohort (< 40 years) generally displaying a more vigorous response compared to the older age groups (40 to < 60 years and ≥ 60 years). However, it is crucial to note that even in older individuals, vaccination induced significant immune responses, emphasizing the need to vaccinate across all age groups to achieve comprehensive protection against COVID-19.
The protection provided by COVID-19 vaccines against infection significantly decreased between 5 to 8 months after receiving the initial vaccination [26,27]. Booster shots for vaccines have been shown to effectively restore protection against infection and are generally considered safe for use in the community [28-30].
Our study had certain limitations as it focused on observing patients from a single center during hospitalization, which may not fully capture the overall nationwide situation of the COVID-19 pandemic. Additionally, the study had a relatively small sample size. However, it is important to note that our study highlighted a significant finding: the levels of antibodies exhibited a consistent decrease after six months, regardless of gender or age group. This decline was notably reversed with the administration of a booster dose. Our results emphasize the necessity for regular booster doses to sustain a strong and protective antibody level.
Conclusion
Our research provides valuable insights into the immune response to SARS-CoV-2 infection and vaccination, considering both gender and age factors. The results underscore the importance of vaccination as a key strategy in developing protective immunity for diverse populations. Tailoring vaccination approaches based on gender differences and considering vaccination in both previously infected and non-infected individuals will aid in optimizing immune responses and combatting the COVID-19 pandemic effectively. Moreover, a booster dose of the COVID-19 vaccine enhances the antibody response.
Author contributions
Md. Shakeel Ahmed was the principal investigator responsible for the study conception, design, validation, review, and editing of the manuscript. Md. Zakir Hossain was responsible for the laboratory staff’s laboratory resources, investigation, and supervision. Md. Mamunur Rashid’s role was the laboratory research methodology, investigation, and validation. Istiak Ahmad’s role was COVID-19 case selection. Md. Zahirul Islam’s role was laboratory research methodology, study, validation, formal data analysis, original draft writing, and editing of the manuscript. Meherab Hossain and Hasan Rabbi assisted in patient specimen collection and laboratory methodology.
The authors thank Rodolphe Merièux Laboratory, Fouzderhat, Chittagong, for providing laboratory facilities, reagents, and technical support throughout the study.
- Amanat F, Stadlbauer D, Strohmeier S, Nguyen THO, Chromikova V, McMahon M, Jiang K, Arunkumar GA, Jurczyszak D, Polanco J, Bermudez-Gonzalez M, Kleiner G, Aydillo T, Miorin L, Fierer DS, Lugo LA, Kojic EM, Stoever J, Liu STH, Cunningham-Rundles C, Felgner PL, Moran T, García-Sastre A, Caplivski D, Cheng AC, Kedzierska K, Vapalahti O, Hepojoki JM, Simon V, Krammer F. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med. 2020 Jul;26(7):1033-1036. doi: 10.1038/s41591-020-0913-5. Epub 2020 May 12. PMID: 32398876; PMCID: PMC8183627.
- Okba NMA, Müller MA, Li W, Wang C, GeurtsvanKessel CH, Corman VM, Lamers MM, Sikkema RS, de Bruin E, Chandler FD, Yazdanpanah Y, Le Hingrat Q, Descamps D, Houhou-Fidouh N, Reusken CBEM, Bosch BJ, Drosten C, Koopmans MPG, Haagmans BL. Severe Acute Respiratory Syndrome Coronavirus 2-Specific Antibody Responses in Coronavirus Disease Patients. Emerg Infect Dis. 2020 Jul;26(7):1478-1488. doi: 10.3201/eid2607.200841. Epub 2020 Jun 21. PMID: 32267220; PMCID: PMC7323511.
- Fedele G, Stefanelli P, Bella A, Fiore S, Pancheri S, Benedetti E, Fabiani C, Leone P, Vacca P, Schiavoni I, Neri A, Carannante A, Simmaco M, Santino I, Zuccali MG, Bizzarri G, Magnoni R, Benetollo PP, Brusaferro S, Rezza G, Ferro A. Anti-SARS-CoV-2 antibodies persistence after natural infection: a repeated serosurvey in Northern Italy. Ann Ist Super Sanita. 2021 Oct-Dec;57(4):265-271. doi: 10.4415/ANN_21_04_01. PMID: 35076416.
- Ortega N, Ribes M, Vidal M, Rubio R, Aguilar R, Williams S, Barrios D, Alonso S, Hernández-Luis P, Mitchell RA, Jairoce C, Cruz A, Jimenez A, Santano R, Méndez S, Lamoglia M, Rosell N, Llupià A, Puyol L, Chi J, Melero NR, Parras D, Serra P, Pradenas E, Trinité B, Blanco J, Mayor A, Barroso S, Varela P, Vilella A, Trilla A, Santamaria P, Carolis C, Tortajada M, Izquierdo L, Angulo A, Engel P, García-Basteiro AL, Moncunill G, Dobaño C. Seven-month kinetics of SARS-CoV-2 antibodies and role of pre-existing antibodies to human coronaviruses. Nat Commun. 2021 Aug 6;12(1):4740. doi: 10.1038/s41467-021-24979-9. PMID: 34362897; PMCID: PMC8346582.
- Wang P, Nair MS, Liu L, Iketani S, Luo Y, Guo Y, Wang M, Yu J, Zhang B, Kwong PD, Graham BS, Mascola JR, Chang JY, Yin MT, Sobieszczyk M, Kyratsous CA, Shapiro L, Sheng Z, Huang Y, Ho DD. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021 May;593(7857):130-135. doi: 10.1038/s41586-021-03398-2. Epub 2021 Mar 8. PMID: 33684923.
- Wilhelm A, Widera M, Grikscheit K, Toptan T, Schenk B, Pallas C, Metzler M, Kohmer N, Hoehl S, Marschalek R, Herrmann E, Helfritz FA, Wolf T, Goetsch U, Ciesek S. Limited neutralisation of the SARS-CoV-2 Omicron subvariants BA.1 and BA.2 by convalescent and vaccine serum and monoclonal antibodies. EBioMedicine. 2022 Aug;82:104158. doi: 10.1016/j.ebiom.2022.104158. Epub 2022 Jul 11. PMID: 35834885; PMCID: PMC9271884.
- Chodick G, Tene L, Patalon T, Gazit S, Ben Tov A, Cohen D, Muhsen K. Assessment of Effectiveness of 1 Dose of BNT162b2 Vaccine for SARS-CoV-2 Infection 13 to 24 Days After Immunization. JAMA Netw Open. 2021 Jun 1;4(6):e2115985. doi: 10.1001/jamanetworkopen.2021.15985. PMID: 34097044; PMCID: PMC8185600.
- Hanrath AT, Payne BAI, Duncan CJA. Prior SARS-CoV-2 infection is associated with protection against symptomatic reinfection. J Infect. 2021 Apr;82(4):e29-e30. doi: 10.1016/j.jinf.2020.12.023. Epub 2020 Dec 26. PMID: 33373652; PMCID: PMC7832116.
- Mushtaq S, Azam Khan MK, Alam Khan MQ, Rathore MA, Parveen B, Noor M, Ghani E, Tahir AB, Tipu HN, Lin B. Comparison of immune response to SARS-COV-2 vaccine in COVID-recovered versus non-infected Individuals. Clin Exp Med. 2023 Feb 21:1–7. doi: 10.1007/s10238-023-01005-4. Epub ahead of print. PMID: 36802308; PMCID: PMC9942049.
- Karachaliou M, Moncunill G, Espinosa A, Castaño-Vinyals G, Rubio R, Vidal M, Jiménez A, Prados E, Carreras A, Cortés B, Blay N, Bañuls M, Pleguezuelos V, Melero NR, Serra P, Parras D, Izquierdo L, Santamaría P, Carolis C, Papantoniou K, Goldberg X, Aguilar R, Garcia-Aymerich J, de Cid R, Kogevinas M, Dobaño C. SARS-CoV-2 infection, vaccination, and antibody response trajectories in adults: a cohort study in Catalonia. BMC Med. 2022 Sep 16;20(1):347. doi: 10.1186/s12916-022-02547-2. PMID: 36109713; PMCID: PMC9479347.
- Anderson M, Stec M, Rewane A, Landay A, Cloherty G, Moy J. SARS-CoV-2 Antibody Responses in Infection-Naive or Previously Infected Individuals After 1 and 2 Doses of the BNT162b2 Vaccine. JAMA Netw Open. 2021 Aug 2;4(8):e2119741. doi: 10.1001/jamanetworkopen.2021.19741. PMID: 34357399; PMCID: PMC8346938.
- Anichini G, Terrosi C, Gandolfo C, Gori Savellini G, Fabrizi S, Miceli GB, Cusi MG. SARS-CoV-2 Antibody Response in Persons with Past Natural Infection. N Engl J Med. 2021 Jul 1;385(1):90-92. doi: 10.1056/NEJMc2103825. Epub 2021 Apr 14. PMID: 33852796; PMCID: PMC8063888.
- Voysey M, Costa Clemens SA, Madhi SA, Weckx LY, Folegatti PM, et al. Oxford COVID Vaccine Trial Group. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021 Mar 6;397(10277):881-891. doi: 10.1016/S0140-6736(21)00432-3. Epub 2021 Feb 19. Erratum in: Lancet. 2021 Mar 6;397(10277):880. PMID: 33617777; PMCID: PMC7894131.
- Shrotri M, Navaratnam AMD, Nguyen V, Byrne T, Geismar C, Fragaszy E, Beale S, Fong WLE, Patel P, Kovar J, Hayward AC, Aldridge RW; Virus Watch Collaborative. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet. 2021 Jul 31;398(10298):385-387. doi: 10.1016/S0140-6736(21)01642-1. Epub 2021 Jul 17. PMID: 34274038; PMCID: PMC8285117.
- Iacobucci G. Covid-19: Antibodies after AstraZeneca and Pfizer vaccines decrease with age and are higher in women, data show. BMJ. 2022 Feb 18;376:o428. doi: 10.1136/bmj.o428. PMID: 35181594.
- Hoque A, Barshan AD, Chowdhury FUH, Fardous J, Hasan MJ, Khan MAS, Kabir A. Antibody Response to ChAdOx1-nCoV-19 Vaccine Among Recipients in Bangladesh: A Prospective Observational Study. Infect Drug Resist. 2021 Dec 19;14:5491-5500. doi: 10.2147/IDR.S335414. PMID: 34984006; PMCID: PMC8702783.
- Speiser DE, Bachmann MF. COVID-19: Mechanisms of Vaccination and Immunity. Vaccines (Basel). 2020 Jul 22;8(3):404. doi: 10.3390/vaccines8030404. PMID: 32707833; PMCID: PMC7564472.
- Hall V, Foulkes S, Insalata F, Kirwan P, Saei A, et al. SIREN Study Group. Protection against SARS-CoV-2 after Covid-19 Vaccination and Previous Infection. N Engl J Med. 2022 Mar 31;386(13):1207-1220. doi: 10.1056/NEJMoa2118691. Epub 2022 Feb 16. PMID: 35172051; PMCID: PMC8908850.
- Tregoning JS, Flight KE, Higham SL, Wang Z, Pierce BF. Progress of the COVID-19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat Rev Immunol. 2021 Oct;21(10):626-636. doi: 10.1038/s41577-021-00592-1. Epub 2021 Aug 9. PMID: 34373623; PMCID: PMC8351583.
- Chirico F, Teixeira da Silva JA. Evidence-based policies in public health to address COVID-19 vaccine hesitancy. Future Virol. 2023 Mar:10.2217/fvl-2022-0028. doi: 10.2217/fvl-2022-0028. Epub 2023 Apr 4. PMID: 37034451; PMCID: PMC10079004.
- Yang J, Vaghela S, Yarnoff B, De Boisvilliers S, Di Fusco M, Wiemken TL, Kyaw MH, McLaughlin JM, Nguyen JL. Estimated global public health and economic impact of COVID-19 vaccines in the pre-omicron era using real-world empirical data. Expert Rev Vaccines. 2023 Jan-Dec;22(1):54-65. doi: 10.1080/14760584.2023.2157817. Epub 2022 Dec 28. PMID: 36527724.
- Kaim A, Siman-Tov M, Jaffe E, Adini B. Effect of a Concise Educational Program on COVID-19 Vaccination Attitudes. Front Public Health. 2021 Nov 30;9:767447. doi: 10.3389/fpubh.2021.767447. PMID: 34917578; PMCID: PMC8669390.
- van Kessel R, Forman R, Milstein R, Mastylak A, Czabanowska K, Czypionka T, Durand-Zaleski I, Hirche A, Krysinska-Pisarek M, Maynou L, Roberts B, Torbica A, Vrangbæk K, Wang Y, Wouters OJ, Mossialos E. Divergent COVID-19 vaccine policies: Policy mapping of ten European countries. Vaccine. 2023 Apr 24;41(17):2804-2810. doi: 10.1016/j.vaccine.2023.03.036. Epub 2023 Mar 22. PMID: 36967287; PMCID: PMC10030332.
- Leier HC, Bates TA, Lyski ZL, McBride SK, X Lee D, Coulter FJ, Goodman JR, Lu Z, Curlin ME, Messer WB, Tafesse FG. Previously infected vaccinees broadly neutralize SARS-CoV-2 variants. medRxiv. 2021 Apr 29:2021.04.25.21256049. doi: 10.1101/2021.04.25.21256049.
- Beck EJ, Hsieh YH, Fernandez RE, Dashler G, Egbert ER, Truelove SA, Garliss C, Wang R, Bloch EM, Shrestha R, Blankson J, Cox AL, Manabe YC, Kickler T, Rothman RE, Redd AD, Tobian AAR, Milstone AM, Quinn TC, Laeyendecker O. Differentiation of Individuals Previously Infected with and Vaccinated for SARS-CoV-2 in an Inner-City Emergency Department. J Clin Microbiol. 2022 Mar 16;60(3):e0239021. doi: 10.1128/jcm.02390-21. Epub 2022 Jan 19. PMID: 35044204; PMCID: PMC8925900.
- Mahase E. Covid-19: Booster vaccine gives "significant increased protection" in over 50s. BMJ. 2021 Nov 17;375:n2814. doi: 10.1136/bmj.n2814. PMID: 34789456.
- Andrews N, Stowe J, Kirsebom F, Toffa S, Sachdeva R, Gower C, Ramsay M, Lopez Bernal J. Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England. Nat Med. 2022 Apr;28(4):831-837. doi: 10.1038/s41591-022-01699-1. Epub 2022 Jan 14. PMID: 35045566; PMCID: PMC9018410.
- Barda N, Dagan N, Cohen C, Hernán MA, Lipsitch M, Kohane IS, Reis BY, Balicer RD. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 2021 Dec 4;398(10316):2093-2100. doi: 10.1016/S0140-6736(21)02249-2. Epub 2021 Oct 29. PMID: 34756184; PMCID: PMC8555967.
- Munro APS, Janani L, Cornelius V, Aley PK, Babbage G, et al. COV-BOOST study group. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet. 2021 Dec 18;398(10318):2258-2276. doi: 10.1016/S0140-6736(21)02717-3. Epub 2021 Dec 2. Erratum in: Lancet. 2021 Dec 18;398(10318):2246. PMID: 34863358; PMCID: PMC8639161.
- Menni C, May A, Polidori L, Louca P, Wolf J, Capdevila J, Hu C, Ourselin S, Steves CJ, Valdes AM, Spector TD. COVID-19 vaccine waning and effectiveness and side-effects of boosters: a prospective community study from the ZOE COVID Study. Lancet Infect Dis. 2022 Jul;22(7):1002-1010. doi: 10.1016/S1473-3099(22)00146-3. Epub 2022 Apr 8. PMID: 35405090; PMCID: PMC8993156.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.




 Save to Mendeley
Save to Mendeley
