Journal of Vaccines and Immunology
Percutaneous Patent Ductus Arteriosus (PDA) closure: When and how to close Coil VS Occluder “step by step” cases report
Nassime Zaoui*, Amina Boukabous, Nabil Irid, Katia Babou and Sabrina Benamara
Cite this as
Zaoui N, Boukabous A, Irid N, Babou K, Benamara S (2023) Percutaneous Patent Ductus Arteriosus (PDA) closure: When and how to close Coil VS Occluder “step by step” cases report. J Vaccines Immunol 9(1): 015-023. DOI: 10.17352/jvi.000056Copyright
© 2023 Zaoui N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Background: The PDA defines the pathological persistence after the birth of a fetal physiological communication between the aorta and the pulmonary artery frequently encountered in preterm infants and whose clinical and hemodynamic consequences depend on the importance of the shunt directly bound to the diameter of the canal.
Percutaneous closure is the most frequent management modality with excellent immediate and long-term results (two modes of closure: using coil or Occluder).
The surgery remains reserved for complex anatomies or associated with other surgical congenital anomalies.
Case presentation: We detail in this document the two methods of percutaneous closure step by step illustrated by pediatric cases. The first case concerns a 7 years old girl of 17 kg weight with a history of heart murmur that presented in the TTE a PDA estimated at 1mm with LV dilation. The second case concerns a 12 years old girl of 30 kg weight with also a history of heart murmur that presented on TTE a PDA of 4.5mm with LV dilation.
Therapeutic intervention: In the first case, we perform a closure with coil 5/5 by a unique femoral arterial approach as a standardized attitude in our center avoiding additional venous access. For the second case, we opted for closure with prosthesis N° 6/8 by a double femoral approach (arterial and venous access).
Outcomes: The follow-up was favorable for both patients, with total sealing of the defect immediately after the procedures that persist during the 6 months of control.
Conclusion: The closure of PDA in children is a challenging procedure whose safety requires a good pre-and per-procedural evaluation allowing the right choice of the method and size of the closing device.
The respect of the different closure stages and the critical per procedural ultrasound and angiographic control reduce the rate of complications making this technique accessible and safe.
In our series of 108 PDA closures by Coil in children, the unique femoral arterial approach is the standardized attitude in the first line in all patients avoiding additional venous access, which allows the Coil release in the basic technique while the arterial access allows opacification and measurement of the channel.
The unique arterial approach has reduced the risk of local complications at the puncture site and the duration of the procedure without difference in closure efficiency and embolization risk.
In our series of 92 PDA closures by Occluder in children the double femoral approach is the standardized attitude for all patients, the venous access allows the device release while the arterial access allows opacification/ measurement of the channel and control device deployment.
Abbreviations
LV: Left Ventricle; NSAIDs: Non-Streoidal Anti-Inflammatory Drugs; PA: Pulmonary Artery; PDA: Persistent Ductus Arteriosus; TTE: Transthoracic Echocardiography; Vmax: Maximum Velocity; WU: Wood Unity
Introduction
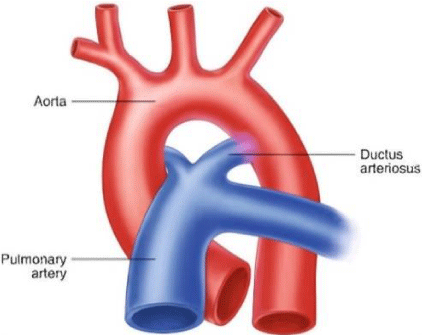
PDA is the persistence of the fetal connection between the aorta and pulmonary artery after birth [1]. Shunting in the PDA is left to right (from the aorta to the the pulmonary artery: Figure 1) [1,2].
It represents 5 to 10% of congenital heart anomalies, with a sex ratio of male: to female at 1:3 [2].
PDA is very common among premature infants (present in about 45% with birth weight < 1750 g and in 70 to 80% with birth weight < 1200 g) [3].
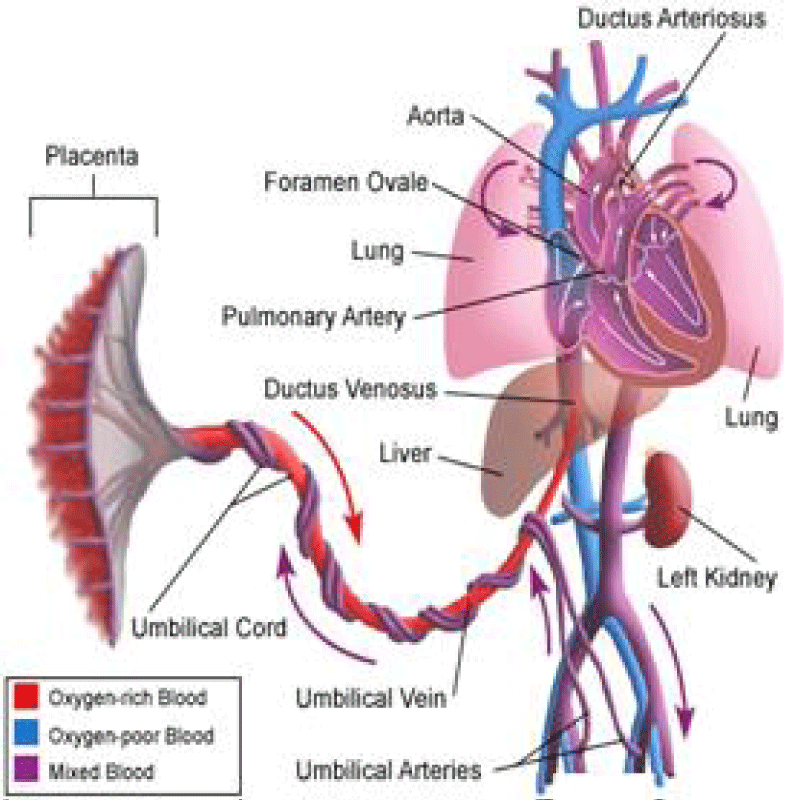
The Ductus arteriosus is a normal connection between PA and the aorta; it is necessary for fetal circulation [3].
At birth, a rise in PaO2 and a decline in prostaglandin cause its closure within the first 15 hours of life [4]. If this normal process does not occur for at least 1 month, it is a PDA (Figure 2) [5,6].
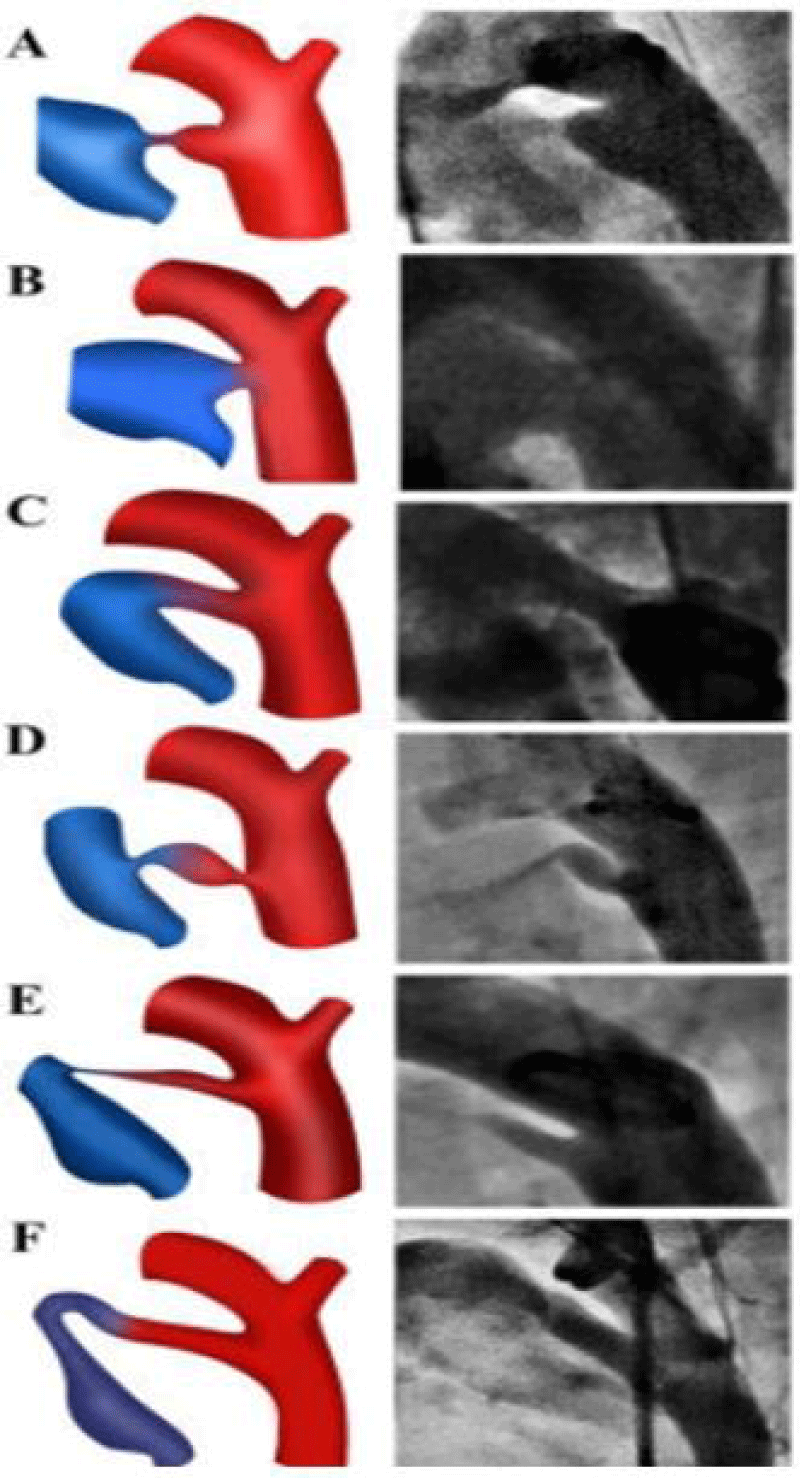
PDA is classified according to Krichenko [7] classification is based on angiographic findings with six types (figure 3).
Familial forms are frequent (multigenic), and other causes and associations are described as maternal smoking, prostaglandins, maternal rubella, life at altitude, and trisomy 21 [6,8].
The symptomatology depends on the PDA size (asymptomatic in small PDAs, while large PDAs are associated with failure to thrive, poor feeding, poor weight gain, frequent respiratory infection, tachycardia, and tachypnea [9]. The clinical examination finds a continuous murmur at the upper left sternal border and bounding pulses (Figure 4) [9,10].
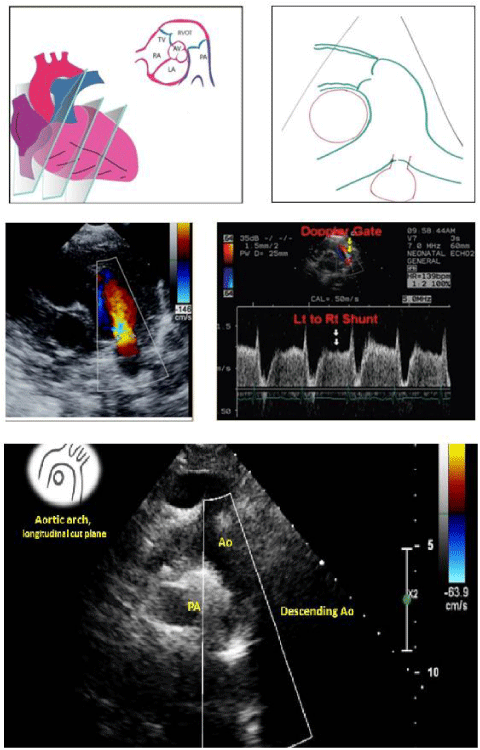
Trans-Thoracic Echocardiography has an important role in PDA; It makes it possible to identify the PDA, specify its anatomy and size (correlation index at 0.73 with angiographic measurement), and quantify the shunt and its impact on the left cavities size and on the pulmonary pressures [10,11] (Figure 5).
About 1/3 of PDAs will close spontaneously, Portsman, et al. introduced Transcatheter closure in 1967 using the conical Ivalon plug [1,3].
According to ESC 2020 guidelines [12] for the management of adult congenital heart disease, PDA closure is indicated in patients with evidence of left ventricle overload and no pulmonary hypertension (class I). Percutaneous closure is the method of choice when technically suitable.
The closure is indicated from the age of 1 year, it is usually performed between 3 and 15 years and remains indicated even in elderly patients.
Medical treatment is reserved for heart failure in premature babies (no indication for prophylactic purposes) by NSAIDs, indomethacin, oral or IV paracetamol (Class I). [13-15].
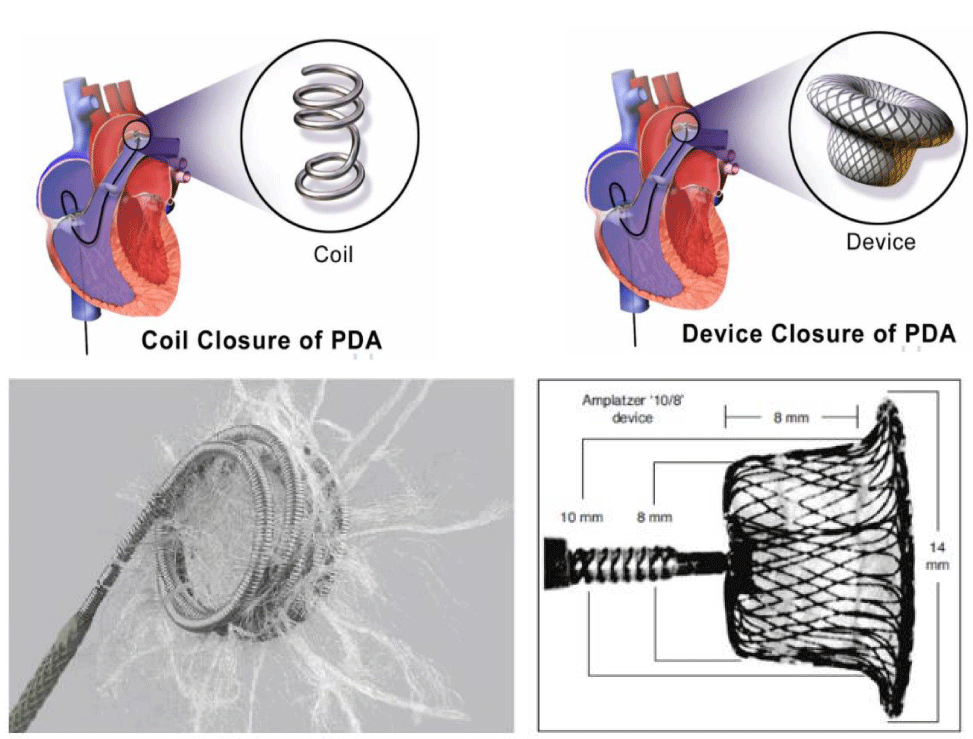
Percutaneous closure [16-19] (Figure 6) is based on Coils for PDAs < 3 mm (Coil diameter is equal or superior 2 times the smallest PDA diameter) and Occluder for PDAs > 3 mm (Occluder size is equal to the smallest PDA diameter + 2 mm).
This procedure is followed by Osler prophylaxis for 6 months in case of any bacterial infection.
The Surgical treatment [20] with a postero-lateral thoracotomy in the 4th left intercostal space by a simple ligature (recanalization risk) or section/Suture is indicated only in case of percutaneous treatment contraindication or association with operable heart disease (Figure 7).
We describe here the percutaneous closure procedures in two children as faithfully as possible with tips and tricks to solve all the difficulties encountered.
Percutaneous PDA closure procedure with the coil (step by step)
- Patient information: Seven years old girls, 17 kg weight followed for heart murmur known since the age of 3 years.
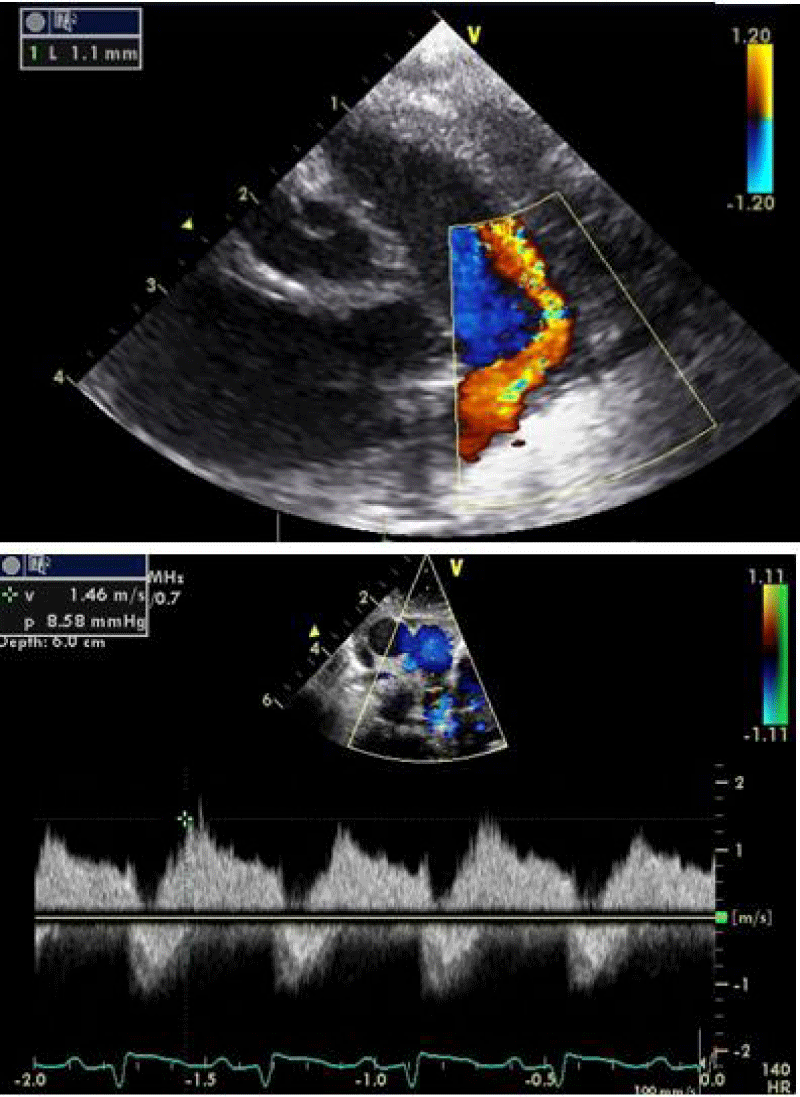
- Clinical findings and diagnostic assessment: TTE identifies a PDA estimated at 1 mm with upper limit size LV without PH (Figure 8).
- Therapeutic intervention:
a- Material:
5Fr radial sheath (on the right femoral artery).
0.035’’ 145 cm J tapped guide wire.
0.035’’ 145 cm straight guide wire.
0.014’’ 180 cm Hydrophilic straight guide wire.
5Fr Judkins Right diagnostic catheter.
5Fr Judkins Right Guiding catheter.
Coil 5/5 and its Flipper (COOK©).
Ultravist 300 contrast.
b- Technique:
We started the procedure by sedating the child under Sevoflutane with a mask and monitoring the heart rate, blood pressure, and oxygen saturation.
Then we proceeded to rigorous disinfection and establishment of sterile fields and punctured the right femoral artery under local anesthesia with Lidocaine 1% using 5F radial sheath then we injected Heparin at a dose of 100 units per Kg (1700 units).
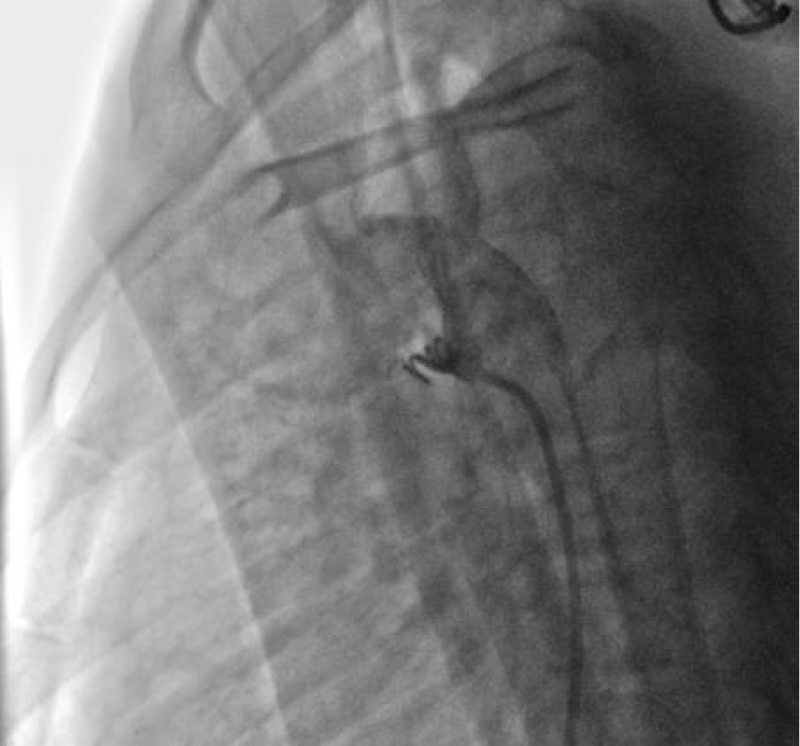
We then introduced a JR 3.5 5F diagnostic catheter on a 0.035’’ wire to the aortic isthmus and then we opacified the channel by manual injections of ULTRAVIST 300 contrast to confirm its diameter by angiographic measurement in profile view (1.3 +/- 0.3 mm) (Figure 9).
Then we took a JR 4 5F guiding catheter closed by an angioplasty Python, we crossed the channel to the pulmonary artery with a 0.014’’ hydrophilic wire and a 0.035’’ straight wire used as a body wire to partially engage the PDA.
The PDA being long with 1.6 mm in diameter, we opted for a coil of 5/5 (5 loops of 5mm) deployed erect in the pulmonary artery after pulling back the guiding catheter, then the central mandrel of the coil is gradually removed to deploy 1.5 loops in the pulmonary artery, 1.5 loops in the channel and 2 loops in the aorta (Figure 10).
An echocardiographic control is carried out at this time to confirm the right coil positioning, the channel closure, and the absence of arterial clutter on both aorta and PA (V max < 2m/s).
We ended up with an angiographic control with contrast injection before delivering the coil by the counter-clockwise rotation of the external part of its delivery system (Figure 11).
A final angiographic and ultrasound control was carried out 10 minutes later in Cath Lab before the removal of the material and manual compression of the right femoral artery.
The procedure skin to skin took 30 minutes.
A compressive bandage was placed for 12 hours. The child was monitored in the awakening room for 2 hours at the end of which she returned to the normal hospital bed.
4- Follow-up and outcomes: An echocardiographic control was carried out 24 hours later at the end of which the exit was authorized with antibiotic prophylaxis in front of any septic gesture for 6 months (Figure 12).
A clinical and echocardiographic follow-up was performed at 1, 3, and 6 months with total closure of the PDA without a residual shunt.
5- Discussion: In our series of 108 PDA closures by Coil in children, the unique femoral arterial approach is the standardized attitude in the first line in all patients avoiding additional venous access, which allows the Coil release in the basic technique [17] while the arterial access allows opacification and measurement of the channel.
The unique arterial approach has reduced the risk of local complications at the puncture site and the duration of the procedure without difference in closure efficiency and embolization risk.
In the case of a minimal residual shunt (restrictive flow > 4m / s), 10 minutes after delivering the coil control is carried out 24 hours then 1 to 3 months after, spontaneous total closure is seen in 100% of cases in our series (5 cases on 108 children closed by coils) (Figure 13).
In the case of a large shunt (non-restrictive flow) after 10 minutes a second coil can be introduced contralaterally (through the pulmonary artery by femoral venous access) either immediately or after a period of one month (2 patients in our series required a second coil to close their PDA, one closed immediately, the second 1 month after) successfully in 100% of cases (Figure 14).
We do not report in our children’s series any cases of coil embolized.
6- Patient perspective: The parents were very satisfied with the result. Their daughter quickly joined the school as well as a normal sports activity and social life.
7- Informed consent: The parents consented to the sharing and publishing of her case and procedure images subject to anonymity.
Percutaneous PDA closure procedure with Occluder device (step by step)
- Patient information: Twelve years old girl, 30 kg weight followed for heart murmur known since the age of 8 years.
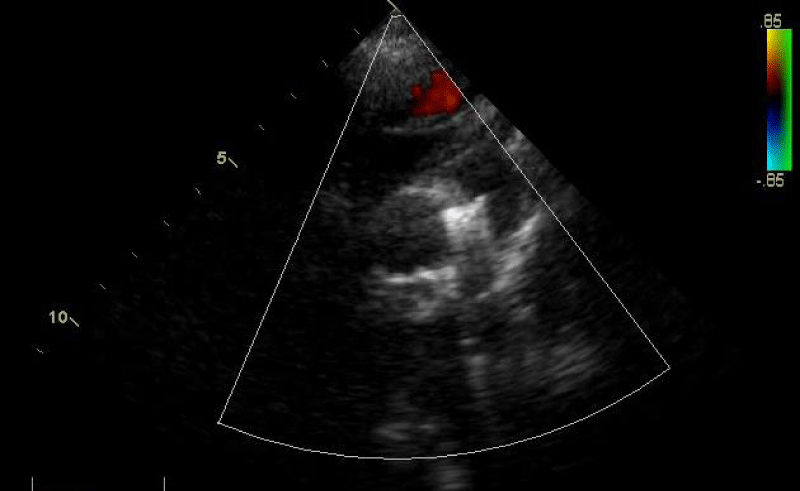
- Clinical findings and diagnostic assessment: TTE identifies a PDA estimated at 4.5 mm with upper limit size LV without PH (Figure 15).
- Therapeutic intervention:
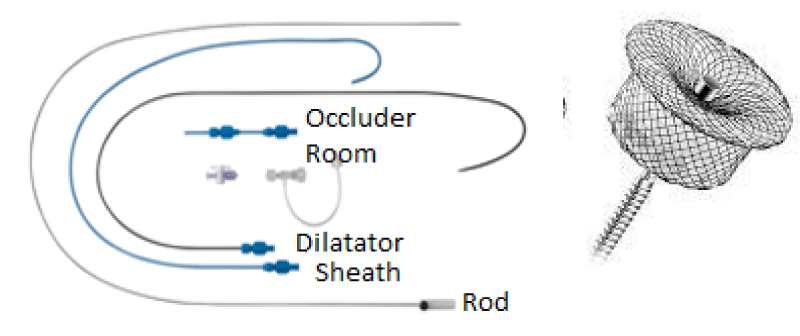
Material: (Figure 16)
5Fr radial sheath (on the right femoral artery).
6Fr radial sheath (on the right femoral vein).
0.035’’ 145 cm J tapped guide wire.
0.035’’ 260 cm hydrophilic straight guide wire.
Two 5Fr Judkins Right diagnostic catheter.
7Fr Delivery system.
PDA Occluder N° 6/8 (ANDRATECH©).
ULTRAVIST 300 contrast.
Technique: We started the procedure by sedating the child under sevoflutane with a mask and monitoring the heart rate, blood pressure, and oxygen saturation.
Then we proceeded to rigorous disinfection and establishment of sterile fields and punctured the right femoral artery under local anesthesia with Lidocaine 1% using a 5F radial sheath.
We then introduced a JR 3.5 5F diagnostic catheter on a 0.035’’ wire to the aortic isthmus and then we opacified the channel by manual injections of ULTRAVIST 300 contrast to confirm its diameter by angiographic measurement in profile view (4.3 +/- 0.4 mm) (Figure 17).
Therefore, we planned to implant a 6/8 PDA Occluder that requires a 7Fr delivery system, for that, we punctured the right femoral vein using a 6fr radial sheath and injected Heparin at a dose of 100 units per Kg (3000 units) then we crossed the right cavities to the pulmonary artery by a JR 3.5 5Fr diagnostic catheter on 0.035’’ wire.
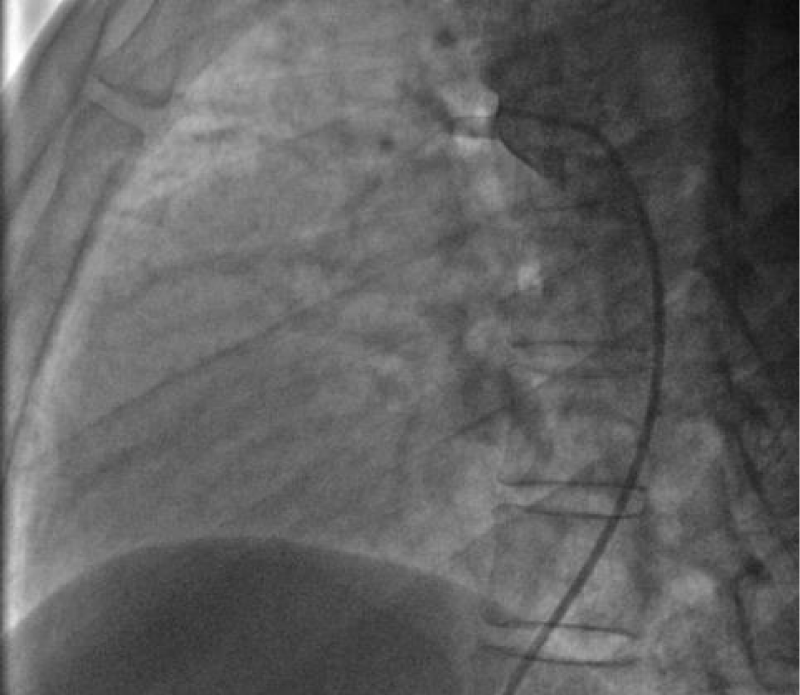
After failing to cross the channel by a straight hydrophilic guide 0.035’’ because of the smallness of the PDA caliber on its pulmonary side we decided to cross it by its aortic side with the hydrophilic straight 0.035’’ 260 cm wire that we exteriorized from the left JR 3.5 5Fr diagnostic catheter through the JR 3.5 5Fr diagnostic catheter mounted on the right on which we introduced the delivery system 7F by the right femoral venous access (Figure 18).
The delivery system is placed from the femoral vein to the right cavities and then through the canal to the aortic isthmus, the prosthesis is debubbled and fixed by screwing on its rod then mounted in its chamber (room) under serum washing and conveyed to the end of the 7F sheath (Figure 19).
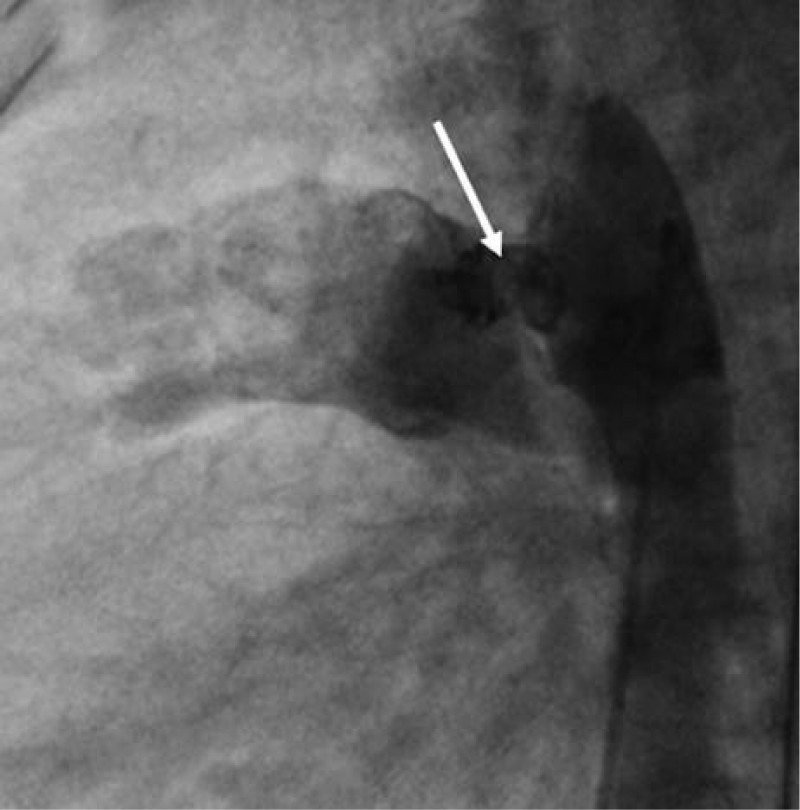
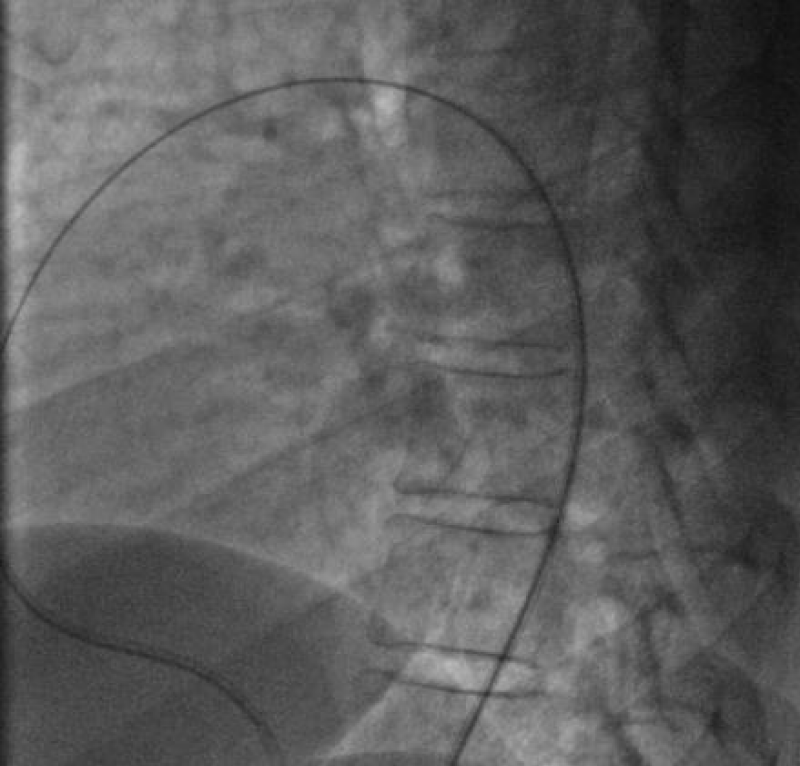
We deployed the distal part of the device in the aortic isthmus and pulled back the entire system to the aortic ampulla under angiographic control (Figure 20).
Traction was, then, maintained on the rod with the removal of the 7Fr sheath until the device was completely deployed into the body of the channel, injections through the JR3.5 5F catheter placed in the aorta were performed close to the device confirming its correct positioning in the aortic ampulla and absence of residual shunt, comforted by an echocardiographic control (Figure 21).
We proceeded to the definitive release of the device by an anti-clockwise rotation of the external part of its rod and we ended up with an echocardiographic and angiographic control with contrast injection (Figure 22).
A final angiographic and ultrasound control was carried out 10 minutes later in Cath Lab before the removal of the material and manual compression of the right femoral artery and vein.
The procedure skin to skin took 40 minutes.
A compressive bandage was placed for 12 hours. The child was monitored in the awakening room for 2 hours at the end of which she returned to the normal hospital bed.
4- Follow-up and outcomes: An echocardiographic control was carried out 24 hours later at the end of which the exit was authorized with antibiotic prophylaxis in front of any septic gesture for 6 months.
A clinical and echocardiographic follow-up was performed at 1, 3, and 6 months with total closure of the PDA without a residual shunt.
5- Discussion: In our series of 92 PDA closures by Occluder in children the double femoral approach is the standardized attitude for all patients, the venous access allows the device release while the arterial access allows opacification/ measurement of the channel and control device deployment [19].
In the case of a minimal residual shunt (restrictive flow > 4m / s), 10 minutes after delivering the device control is carried out 24 hours than 1 to 3 months after, spontaneous total closure is seen in 100% of cases in our series (3 cases out of 92 children closed by Occluder).
In the case of a large shunt (non-restrictive flow) after 10 minutes a coil can be introduced contralaterally (through the aorta by femoral arterial access) either immediately or after a period of one month (1 case sealed by a Coil added 3 months avec the first procedure) successfully in 100% of cases.
We deplore in our children’s series (at the beginning of our experiment) a case of Occluder 6/8 embolization recovered by last in a delivery system 12Fr due to a mismatch between the size of the channel and the prosthesis.
6- Patient perspective: The parents were very satisfied with the result. Their daughter quickly joined the school as well as a normal physical activity and social life.
7- Informed consent: The parents consented to the sharing and publishing of her case and procedure images subject to anonymity.
Conclusion
The closure of PDA in children is a challenging procedure whose safety requires a good pre-and perprocedural evaluation allowing the right choice of the method and size of the closing device.
The respect of the different closure stages and the critical perprocedural ultrasound and angiographic control reduce the rate of complications making this technique accessible and safe.
What we know about it
PDA is a very common congenital heart disease, its closure is most often percutaneous with excellent results in the short, medium and long term and a very low rate of complication.
The management of the puncture point in this situation remains delicate and requires great concentration, especially in children.
What this clinical case adds
General anesthesia seems to us to be a reassuring attitude in children for percutaneous procedures.
In the coil closure procedure, the reduction of the approach to single femoral access by the abundance of venous access reduces the rate of local complications without harming the success rate of the procedure.
In the case of a minimal residual shunt (restrictive flow > 4m / s), 10 minutes after delivering the coil or the device control is carried out 24 hours than 1 to 3 months after, spontaneous total closure is seen in the majority of cases (100% of cases in our series).
In the case of a large shunt (non-restrictive flow) 10 minutes after delivering the coil or the device, a coil can be introduced contralaterally to the first access either immediately or after a period of one month allowing closing successfully the defect in the majority of cases.
Procedure videos are available on our YouTube channel: UCUps-8DbHFB8EN_ARUCpeZg.
Ethics approval and consent to participate
The parents of both children consented to their children undergoing their procedures.
Consent for publication
The parents consented to the sharing and publication of data, images, and results.
Availability of data and material
The images presented during this work are available from the corresponding author upon reasonable request.
Authors’ contributions
NZ was responsible for the realization of the percutaneous procedures and participated in the writing of the manuscript.
AB participated in the percutaneous procedures and the realization of echocardiography.
NI participated in the realization of the percutaneous procedures and in the writing of the manuscript.
KB participated in the realization of echocardiographies and in the writing of the manuscript.
SB participated in the follow-up of the two patients during and after hospitalization.
All authors read and approved the final manuscript.
We thank our paramedics who participated in the percutaneous procedure.
- Schneider DJ, Moore JW. Patent ductus arteriosus. Circulation. 2006 Oct 24;114(17):1873-82. doi: 10.1161/CIRCULATIONAHA.105.592063. PMID: 17060397.
- Gillam-Krakauer M, Mahajan K. Patent Ductus Arteriosus. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2022: https://www.ncbi.nlm.nih.gov/books/NBK430758/
- Heymann MA, Creasy RK, Rudolph AM. Quantitation of blood flow patterns in the foetal lamb in utero. In: Proceedings of the Sir Joseph Barcroft Centenary Symposium: Foetal and Neonatal Physiology. Cambridge, UK: Cambridge University Press; 1973: 129–135.
- Evans NJ, Archer LN. Postnatal circulatory adaptation in healthy term and preterm neonates. Arch Dis Child. 1990 Jan;65(1 Spec No):24-6. doi: 10.1136/adc.65.1_spec_no.24. PMID: 2306130; PMCID: PMC1590177.
- Reller MD, Ziegler ML, Rice MJ, Solin RC, McDonald RW. Duration of ductal shunting in healthy preterm infants: an echocardiographic color flow Doppler study. J Pediatr. 1988 Mar;112(3):441-6. doi: 10.1016/s0022-3476(88)80333-0. PMID: 2964518.
- Campbell M. Natural history of persistent ductus arteriosus. Br Heart J. 1968 Jan;30(1):4-13. doi: 10.1136/hrt.30.1.4. PMID: 5637557; PMCID: PMC459200.
- Krichenko A, Benson LN, Burrows P, Möes CA, McLaughlin P, Freedom RM. Angiographic classification of the isolated, persistently patent ductus arteriosus and implications for percutaneous catheter occlusion. Am J Cardiol. 1989 Apr 1;63(12):877-80. doi: 10.1016/0002-9149(89)90064-7. PMID: 2929450.
- RECORD RG, McKEOWN T. Observations relating to the aetiology of patent ductus arteriosus. Br Heart J. 1953 Oct;15(4):376-86. doi: 10.1136/hrt.15.4.376. PMID: 13093871; PMCID: PMC503838.
- Davis P, Turner-Gomes S, Cunningham K, Way C, Roberts R, Schmidt B. Precision and accuracy of clinical and radiological signs in premature infants at risk of patent ductus arteriosus. Arch Pediatr Adolesc Med. 1995 Oct;149(10):1136-41. doi: 10.1001/archpedi.1995.02170230090013. PMID: 7550818.
- Skelton R, Evans N, Smythe J. A blinded comparison of clinical and echocardiographic evaluation of the preterm infant for patent ductus arteriosus. J Paediatr Child Health. 1994 Oct;30(5):406-11. doi: 10.1111/j.1440-1754.1994.tb00689.x. PMID: 7833075.
- Singh Y, Katheria A, Tissot C. Functional Echocardiography in the Neonatal Intensive Care Unit. Indian Pediatr. 2018 May 15;55(5):417-424. PMID: 29845957.
- Baumgartner H, De Backer J, Babu-Narayan SV, Budts W, Chessa M, Diller GP, Lung B, Kluin J, Lang IM, Meijboom F, Moons P, Mulder BJM, Oechslin E, Roos-Hesselink JW, Schwerzmann M, Sondergaard L, Zeppenfeld K; ESC Scientific Document Group. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur Heart J. 2021 Feb 11;42(6):563-645. doi: 10.1093/eurheartj/ehaa554. PMID: 32860028.
- Mitra S, Florez ID, Tamayo ME, Mbuagbaw L, Vanniyasingam T, Veroniki AA.. Association of placebo, indomethacin, ibuprofen, and acetaminophen with closure of hemodynamically significant patent ductus arteriosus in preterm infants. 2020; 9(4): 5-14.
- Erdeve O, Yurttutan S, Altug N, Ozdemir R, Gokmen T, Dilmen U, Oguz SS, Uras N. Oral versus intravenous ibuprofen for patent ductus arteriosus closure: a randomised controlled trial in extremely low birthweight infants. Arch Dis Child Fetal Neonatal Ed. 2012 Jul;97(4):F279-83. doi: 10.1136/archdischild-2011-300532. Epub 2011 Dec 5. PMID: 22147286.
- Lewis TR, Shelton EL, Van Driest SL, Kannankeril PJ, Reese J. Genetics of the patent ductus arteriosus (PDA) and pharmacogenetics of PDA treatment. Semin Fetal Neonatal Med. 2018 Aug;23(4):232-238. doi: 10.1016/j.siny.2018.02.006. Epub 2018 Feb 24. PMID: 29510900; PMCID: PMC6098727.
- Iwashima S, Satake E, Uchiyama H, Seki K, Ishikawa T. Closure time of ductus arteriosus after birth based on survival analysis. Early Hum Dev. 2018 Jun;121:37-43. doi: 10.1016/j.earlhumdev.2018.05.003. Epub 2018 May 10. PMID: 29754023.
- P S, Jose J, George OK. Contemporary outcomes of percutaneous closure of patent ductus arteriosus in adolescents and adults. Indian Heart J. 2018 Mar-Apr;70(2):308-315. doi: 10.1016/j.ihj.2017.08.001. Epub 2017 Aug 9. PMID: 29716712; PMCID: PMC5993916.
- Baruteau AE, Hascoët S, Baruteau J, Boudjemline Y, Lambert V, Angel CY, Belli E, Petit J, Pass R. Transcatheter closure of patent ductus arteriosus: past, present and future. Arch Cardiovasc Dis. 2014 Feb;107(2):122-32. doi: 10.1016/j.acvd.2014.01.008. Epub 2014 Feb 20. PMID: 24560920.
- Delaney JW, Fletcher SE. Patent ductus arteriosus closure using the Amplatzer® vascular plug II for all anatomic variants. Catheter Cardiovasc Interv. 2013 Apr;81(5):820-4. doi: 10.1002/ccd.24707. Epub 2012 Dec 6. PMID: 23074167.
- Weisz DE, Giesinger RE. Surgical management of a patent ductus arteriosus: Is this still an option? Semin Fetal Neonatal Med. 2018 Aug;23(4):255-266. doi: 10.1016/j.siny.2018.03.003. Epub 2018 Mar 7. PMID: 29636280.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.












 Save to Mendeley
Save to Mendeley
