Journal of Vaccines and Immunology
Validity of Enzyme-Linked Immunosorbent Assay (ELISA) as a correlate of protection of meningococcal C conjugated vaccine in adolescents with HIV infection
Daniela Vinhas Bertolini1*, Luciana Scarlazzari Costa1, Bruno Stuart de Castro2, Tadeu Pernichelli3, Inneke Marie van der Heijden2,4, Silvia Figueiredo Costa2, Helena Keiko Sato5 and Heloísa Helena de Sousa Marques6
2Medical Research Laboratory 54 (LIM-54, Laboratory for Medical Research 54), Department of Infectious Diseases, Hospital das Clínicas University of São Paulo, São Paulo, Brazil
3Laboratory of Clinical Chemistry (Laboratory of Clinical Chemistry), Santa Casa da Misericórdia de São Paulo, Brazil
4Department of Pathology, Discipline of Microbiology and Immunology, ABC Medicine School, FMABC, Santo Andre, Brazil
5Immunization Division of the São Paulo State, Department of Health, São Paulo, Brazil
6Children’s Institute, Hospital das Clínicas, University of São Paulo, Department of Pediatric Infectious Diseases, São Paulo, Brazil
Cite this as
Bertolini DV, Costa LS, De Castro BS, Pernichelli T, Van der Heijden IM, et al. (2020) Validity of Enzyme-Linked Immunosorbent Assay (ELISA) as a correlate of protection of meningococcal C conjugated vaccine in adolescents with HIV infection. J Vaccines Immunol 6(1): 001-007. DOI: 10.17352/jvi.000028The immunological response to meningococcal C conjugated vaccine can be evaluated by two different tests: the serum bactericidal antibody assay (SBA), which evaluates the qualitative bactericidal capacity of the antibodies, and the Enzyme-Linked Immunosorbent Assay (ELISA), which quantifies the specific serogroup C meningococcal immunoglobulin-G. Incompatibilities between the results of both tests have been reported. Technically, ELISA is simpler, safer, and easier to reproduce when compared to the SBA; thus, an assessment of the validity of ELISA as a protective correlate compared to the SBA gold standard should be performed. This study tested the validity of ELISA for the evaluation of the protective response to the C meningococcal conjugate vaccine in adolescents with HIV compared with the gold standard SBA, to evaluate the reliability of ELISA to infer the protection of the individual.
Blood samples of 92 individuals were analyzed (43 HIV+ and 49 HIV−). We observed a positive intraclass correlation coefficient between the ELISA and SBA responses for HIV+ after vaccination (ricc=0.97; 95% CI, 0.91 to 0.99), and for HIV− before vaccination (ricc=0.98; 95% CI, 0.95 to 0.99). In the HIV+ group, the sensitivity and specificity were both 100% for SBA, and 93.5% and 91.6%, respectively, for ELISA. The observed concordance between the tests suggests that in the HIV+ group the measures of antibodies by ELISA could be extrapolated to predict an individual’s protection, suggesting ELISA’s reliability. Until now, ELISA was unable substitute the gold standard SBA. Further studies are necessary to reproduce our findings.
Introduction
The evaluation of the clinical effectiveness of meningococcal vaccines is a difficult task due to the relative low incidence of meningococcal disease in most countries. Therefore, immunogenicity studies based on serum markers as correlates of protection are considered adequate to infer the effectiveness of the vaccine, and have been used for vaccine licensing by regulatory authorities in several countries [1].
Two techniques are currently used to evaluate the antibodies response to meningococcal vaccines: The Serum Bactericidal Antibody Assay (SBA), which qualitatively evaluates the bactericidal capacity of the antibodies, and the enzyme-linked immunosorbent assay (ELISA), which quantifies the specific meningococcal serogroup C Immunoglobulin-G (IgG) [2,3].
In healthy patients, the gold standard correlate of protection against meningococcal C infection is the SBA titer≥4 with human complement, or SBA≥8 with baby rabbit complement [4-8]. Other authors recommend considering SBA titers≥128 or a fourfold increase of the SBA titers after vaccination [3]. Immunity also can be determined by ELISA antibody titers≥2mcg/mL after vaccination [9-12].
When compared with ELISA, the standardization and execution of SBA involves greater complexity, which affects its reproducibility and may explain the variability of the results obtained by different laboratories [13,14]. Another difficult factor is the problem of sourcing human complement and the variability inherent with this material. Besides the greater difficulties, the SBA test requires the manipulation of the bacteria, posing inherent risks for laboratory staff. The ELISA test, on the other hand, is simpler, safer, and easier to reproduce; it is highly accurate, but has a limited capacity to detect different antigenic varieties. Therefore, due to safety and technical issues, the reliability of ELISA for the determination of the immune response test must be evaluated [15.16]. Discrepancies between ELISA and SBA results have been previously reported. Studies on polysaccharide and conjugate meningococcal vaccines conducted with healthy patients have shown that the extrapolation of the concentration of serum anti-capsular antibodies to predict the bactericidal capacity, and to define the individual’s protection, may be limited [2,15,17-19]. The definitions of protection correlates for immunosuppressed patients and the correlation between ELISA and SBA in these patients have yet to be described.
In this study, we aimed to test the validity of ELISA for the evaluation of the protection response to the meningococcal C conjugate vaccine in adolescents with HIV infection, comparing this to the gold standard SBA. We also performed the same assessment of validity in healthy adolescents, analyzing whether there were differences between the groups. These measurements will make it possible to evaluate if the ELISA test shows the reliability to infer the protection of the individual produced by the meningococcal C conjugate vaccine.
Material and methods
Samples
We used the samples of a previous study (a re-analysis of the same samples)-a clinical trial that evaluated the immunologic response to the meningococcal C conjugate vaccine in adolescents with HIV infection, comparing the response with healthy adolescents [20]. In summary, the patients received the meningococcal C conjugated vaccine-CRM197 (meningococcal C oligosaccharide conjugated with protein CRM197 of Corynebacterium diphtheriae, Chiron/Novartis Vaccines, Siena, Italy) and had their antibody titers determined by SBA and ELISA before vaccination and 30 days after. The gold standard criteria of protection were post vaccinal SBA titers ≥ 8 and a four fold increase of SBA titers from pre- to post-vaccination. Patients who did not meet the post-vaccinal protection criteria received a second dose of the vaccine and had their antibodies titers measured 30 days after the re-vaccination.
SBA
The bactericidal activity of the anti-meningococcal serogroup C antibodies was measured by the SBA test, using an external source of complement (3- to 4-week baby rabbit complement sterile, Pel-Freez Biologicals Division, AR). This test was standardized at a reference laboratory in Brazil. Internationally accepted protocols, based on two studies by Borrow et al., and one by Maslank et al., were used [13,14,16]. For preparation of the inoculum, we used a serogroup C meningococcal strain (C11 strain, phenotype C:16:P1.7-1,1) obtained from Centers for Disease Control and Prevention (CDC). For the SBA test, lyophilized bacteria were cultivated in BHI broth (Brain Heart Infusion, BD Difco Laboratories, Detroit, MI) and further isolated in Mueller-Hinton agar with 5% blood (MHBA). Pure cultures were stored in Mueller-Hinton broth (BD Difco Laboratories) containing 15% of glycerol and frozen to-70oC until they were used. Each assay of SBA was carried out with a fresh aliquot of the microorganism. The complement used in this assay was defrosted immediately before use at 37ºC. Thawed complement aliquots were not reused. Both active and inactive forms of the complement were used in the test. For this reason, the bacteria’s complement-mediated death was checked in each test. The SBA assays were performed twice, and four serum samples of two different patients were tested in each microplate (the pre- and post-vaccine samples). Four controls were performed in each microplate:
(i) The complement activity, (ii) The antigen viability, (iii) the verification of non complement-mediated death, and (iv) The positive controls serum.
The complement and the serum samples were previously inactivated at 56oC for 30 minutes to avoid unexpected reactions or interactions of other complement pathways.
The SBA tests were carried out in different stages: (i) Preparation of the bacterial inoculum (suspension containing 5×103 UFC/mL), (ii) Serial 1:2 dilutions of the sera (samples and controls), (iii) Addition of the bacterial suspension and active complement into the microplates, (iv) Addition of specific Agar (Tryptone Soy Agar broth plus 0.9% of Noble Agar and 0.002mL/L of VCN solution: vancomycin, colistin, and nystatin) into all the wells of the microplate, and (v) Microscopy reading and interpretation of the bacterial growth, which were manually performed by the same examiner each time. The serum bactericidal titer was defined as the last serum dilution that led to the death of at least 50% of the inoculums after incubation for 60 minutes, when compared with the control serum at time zero [4]. The results were registered on a Microsoft Excel spreadsheet and typing consistency was performed to verify typing or measurement errors.
ELISA
The detection and quantification of the IgG antibodies were done by “in house” ELISA (using no commercial kits) according to the technique standardized in a reference laboratory in Brazil. Internationally accepted protocols were used, based on the studies of Gheesling et al., Holder et al., and Elie et al., [21-23].
The ELISA assay was performed in three phases: (i) Standardization: The ideal concentration of the antigen the Neisseria meningitidis serogroup C capsular polysaccharide (National Institute for Biological Standards and Control-NIBSC-Potters Bar, Herts, UK) was diluted in methylated human serum albumin (mHSA of NIBSC, UK) to obtain a suspension-Ps-mHSA-at a concentration of 5 ug/mL, and the solution was used for the sensitization of the microplate (Immulon 2HB Costar High Binding) and the tested samples. One hundred microliters of the suspension were distributed in the wells of the microplate and incubated for approximately 18 hours in a humid chamber at 4ºC. Further, the microplate was washed with a 1% PBS-Brij-35 solution and blocked with 200 μL of serum buffer (bovine serum 5%, Brij-35 0.1% diluted in PBS sterile solution) for 1 hour in a humid chamber at room temperature. (ii) Aspiration of the whole blockade solution from the plate. The reference (anti meningococcal human reference serum CDC 1992, NIBSC) and the control sera (CDC1992 and CDC900268, respectively) were diluted at 1/50, and the samples were diluted at 1/100. The serial dilution of the samples and reference sera were performed as follows: 100 μL of buffer serum (the same used for the blockade) and 200 μL of the reference dilutions and samples were added to each well. After homogenization, 100 μL of the solution was sequentially transferred until the target dilution was reached. Four dilutions were made for the samples and seven dilutions for the reference sera. The solutions were incubated for approximately 18 hours in a humid chamber at 4ºC. (iii) The plate was washed, and 100 μL of prepared conjugate, human anti-IgG labelled with alkaline phosphatase (Sigma-Aldrich A-3187, specific for the gamma chain and against all IgG subclasses) in a 1/6000 dilution were distributed in each well of the microplate and incubated for 2 hours in a humid chamber at room temperature. The plate was washed again and 100 μL of the revealing substrate (p-Nitrophenyl phosphate-PNPP-Sigma-Aldrich N9389) was added to each well. The microplate was incubated at room temperature for approximately 30 minutes, and a spectrophotometry reading was performed at 405 nm.
The data of the standard curves of the samples and controls were registered in a Microsoft Excel spreadsheet, then analyzed and validated according to studies that used a similar methodology for the Neisseria meningitidis capsule (A, C, Y, W-135 serogroups) [21-23]. After typing the data in a Microsoft Excel spreadsheet, the consistency of the typing was performed in order to verify typing or measurement errors.
Statistical analysis
The qualitative variables related to vaccine response were expressed using percentages and chi-square tests, or Fisher’s exact test for comparison between groups (HIV+/HIV-). The quantities of SBA titers and ELISA concentrations were described by an error bar graph (means and respective confidence intervals [CIs]), considering the groups (HIV+/HIV) and the time of the research (pre- and post-vaccination).
The variables related to the SBA titration and ELISA concentrations were categorized according to the protection cut-off points, >=8 for SBA and >=2 for ELISA.
The agreement between the methods to evaluate the response to the vaccine was expressed by the intraclass correlation coefficient, the sensitivity and specificity parameters, and the receiver operating characteristic (ROC) curves, with their respective areas under the curve (AUC). The statistical program used was R-Studio version 0.99.484. The level of significance was 0.05.
Results
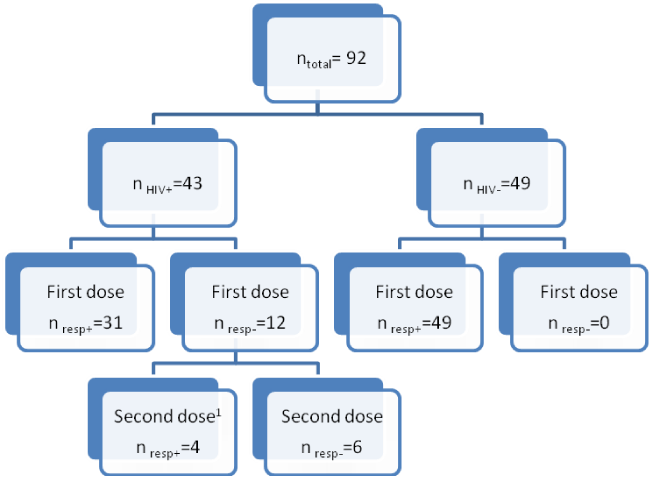
We analyzed the samples of 92 individuals, 43 HIV+ and 49 HIV-patients. Protective immunity (post-vaccinal SBA titers ≥ 8 and a four-fold increase of SBA titers from pre- to post-vaccinal phase) after a single dose of the vaccine was observed in 31 (72.1%) of the HIV+ patients and in 100% of the HIV− patients. Twelve HIV+ patients did not reach protective immunity of the first dose. Of these, one patient was lost to follow up and another patient was excluded due to pregnancy. After revaccination of the 10 non-responders, only four (40%) reached protective antibodies levels (Figure 1).
Sample diagram
The groups were heterogeneous with respect to the response to the vaccine; different proportions were found in relation to SBA titers pre- and post-vaccination, four-fold rise, and vaccine response (Table 1).
Description of the vaccine response by group Pre-vaccination, SBA titers ranged from 4.48 to 35.43 in the HIV+ (geometric mean titer=19.95) and from 13.09 to 66.83 in the HIV- (geometric mean titer=39.96). Postvaccination, SBA titers ranged from 266.51 to 734.14 in the HIV+ (geometric meassn titer=500.33) and from 2019.10 to 3727.73 in the HIV- (geometric mean titer=2873.47) (Figure 2).
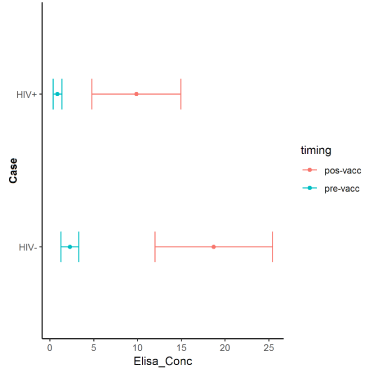
Pre-vaccination, the ELISA levels ranged from 0.36 to 1.36 mcg/mL (geometric mean concentration=0.86) in the HIV+ patients, and from 1.26 to 3.29 mcg/mL (geometric mean=2.27) in the HIV-group. Post-vaccination, the ELISA levels ranged from 4.75 to 14.97mcg/mL (geometric mean=9.86) in the HIV+ and from 11.98 to 25.42mcg/mL (geometric mean=18.71) in the HIV-group (Figure 3).
The intraclass correlations coefficients between the SBA titers and ELISA levels are showed in Table 2. Significant correlations in groups and between methods were found for HIV+patients at post-vaccination time and for HIV- at pre-vaccination time. At the time of pre-vaccination, the correlation between the methods in the HIV+group was weak (0.08). When considering the total of the sample, the correlations were significant in both the pre- and post-vaccination times of the research (Table 2).
The sensitivity and specificity parameters of the methods were evaluated at the time after the vaccine, respecting the cut-off points already established. It was verified that the SBA has a sensitivity and specificity of 100% in the HIV+group, and showed 100% sensitivity in the HIV-group. The ELISA method showed sensitivity and specificity above 90% in the HIV+group, and in the HIV− group all patients were correctly classified (Table 3).
Observing the ROC curve for the post-vaccination period in HIV+, the better diagnostic power of the SBA method was verified (AUC=0.99) when compared to the ELISA method; however, ELISA also had a good diagnostic power, which was verified by its AUC (0.96) (Figure 4).
For the HIV+group who received the second vaccine dose (n=10), the intra-class correlation coefficient between SBA and ELISA was 0.98 (95% CI, 0.48 to 0.99), showing a significant correlation between the techniques for this group.
We re-evaluated both groups for the same parameters, excluding patients who had previously been vaccinated with polysaccharide meningococcal vaccines, with results similar to those of the total sample for both intraclass correlations and sensitivity and specificity parameters, and the area under the ROC curve, considering the two methods that were tested (ELISA and SBA). The sensitivity and specificity for SBA were both 100%, and the sensitivity and specificity for ELISA were 96.4% (ROC curve AUC 0.99) and 90.9% (Roc curve AUC 0.95), respectively.
Discussion
As previously described, technical and safety issues related to the performance of the SBA make it desirable to analyze the reliability of ELISA in the evaluation of the vaccine response to the meningococcal C conjugate vaccine. All the published studies on the correlation between ELISA and SBA evaluated healthy populations. While the majority have shown acceptable correlations between the techniques, such findings are not consistent in the literature. Factors, such as age, the absence of quantification of IgM antibodies by ELISA, and the distribution of the IgG subclass, can influence the results of the correlations [2,15,17,24]. No prior studies have evaluated the correlations between SBA and ELISA in immunocompromised patients.
Our study showed a positive correlation between the ELISA and SBA techniques for HIV+ patients at the time post-vaccination. The same correlation was observed in the group of re-vaccinated patients. The results of the sensitivity and specificity of the SBA>=8 parameters and the elevation of SBA titration in 4× (considered gold standard and protection criteria adopted in the study) were excellent in the HIV+group (100% and 100%, respectively). The same parameters were evaluated in relation to ELISA, showing results above 90%, thus presenting this method as an interesting possibility of evaluation of the vaccine response within this group. These results were reaffirmed when the ROC curve was evaluated, which demonstrated ELISA as a test with good diagnostic power (AUC 0.96). For HIV-patients, good correlations were found between the tests, especially at the pre-vaccination time (sensitivity rates of 100%) with both tests showing excellent performance in evaluating the vaccine response.
Published studies have shown a possible hyporesponsiveness to meningococcal C conjugated vaccine in patients previously vaccinated with a polysaccharide vaccine [25]. The immune mechanisms involved in the hyporesponsiveness are still unknown; however, some possible causes, or immune mechanisms, have been studied to justify this hyporesponsiveness, but there have been no definitive conclusions. The clinical impact of this hyporesponsiveness has not yet been determined [26]. The possibility of hyporesponsiveness could cause a change between the correlations studied. A new evaluation of both groups was made, excluding previously vaccinated patients, with no differences in the correlations or in the sensitivity and specificity parameters, showing that this fact had no influence on the analysis.
The choice of the ELISA technique used also can influence the correlations, depending on the avidity of the antibodies evaluated. High avidity antibodies are predominant in healthy individuals and are more active than the low avidity antibodies in inducing the complement-mediated bactericidal activity and opsonization. However, some authors state that, in adequate concentrations, low avidity antibodies show a good correlation with the bacterial lysis measured by SBA. The standard ELISA test-the technique adopted in this study-detects mostly low avidity antibodies. Some studies in healthy individuals used a modified ELISA technique, which better detects high avidity antibodies and improves the correlations between the ELISA and the SBA techniques [15,18,19]. New studies should be conducted with HIV infected patients using a modified ELISA to assess the avidity of the anti-meningococcal antibodies in this group.
Studies that evaluated the antibody response to measles in HIV patients, and to the HIV 318 infection itself, have shown that the avidity of the antibodies is damaged by the HIV infection, with a predominance of low avidity antibodies. This can be explained by the destruction of the plasmocytes and B cells, and the dysfunction of the terminal differentiation of the B cells, among other possible reasons [27,28]. The predominance of low avidity antibodies might explain the improved performance of the standard ELISA and the good correlations found in HIV+patients. Our findings on the correlation between the SBA and ELISA techniques suggest a significant correlation between HIV+patients at the time post-vaccination and HIV−pre-vaccination. The results of the correlation between HIV+at the post-vaccination point, together with the sensitivity and specificity findings of the tests, present evidence of agreement between the methods. Thus, we can conclude that the ELISA method is reliable for use in evaluating the vaccine response in the HIV+ group, when it is not possible to use the gold standard in the study population. The extrapolation of the anti meningococcal antibodies measured by standard ELISA could define the individual’s protection. Further studies, with a specific sample designed to obtain correlation between SBA and ELISA (standard and modified) and avidity antibodies, are necessary to evaluate whether the ELISA technique could be adopted in the future as a substitute for or a new option of the gold standard-the SBA-in the evaluation of antibody responses to the meningococcal-C vaccine in HIV+patients.
We thank the patients who participated in the study, their parents/guardians, plus the nurses and other staff members for their helpful cooperation. The authors also acknowledge Dr. Mariliza Henrique da Silva and Adriana Balduíno de Azevedo for their support and encouragement.
We also thank the collaboration of Centers for Disease Control and Prevention (CDC), Division of Bacterial Diseases, Meningitis and Vaccine-Preventable Diseases Branch, Dr. George Carlone, Chief of the Laboratory, who kindly provided the meningococcal serogroup C strain and the control sera used for the standardization of the SBA test in our laboratory.
Ethical considerations
This study was approved by the Committee of Ethics on Research of the participant centers, and informed consent was obtained from all adult patients, or children’s legal guardians.
Funding
This study was funded by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazilian National Council for Scientific and Technological Development; Grant No. 478687/2008-7).
- Vipond C, Care R, Feavers IM (2012) History of meningococcal vaccines and their serological correlates of protection. Vaccine 30S: B10–B17. Link: http://bit.ly/31kBgHU
- Sikkema DJ, Friedman KE, Corsaro B, Kimura A, Hildreth SW, et al. (2000) Relationship between serum bactericidal activity and serogroupspecific immunoglobulin G concentration for adults, toddlers and infants immunized with Neisseria meningitidis serogroup C vaccines. Clin Diagn Lab Immunol 7: 764-768. Link: http://bit.ly/3b5WrC2
- Centers for Disease Control and Prevention (CDC) (2005) Morbidity and Mortality Weekly Report. Prevention and Control of Meningococcal Disease: Recommendations of the Advisory Committee on Immunization Practices (ACIP). May 27, 2005/54 (n. RR-7). Link: http://bit.ly/31kT0TD
- Borrow R, Andrews N, Goldblatt D, Miller EL (2001) Serological basis for use of meningococcal serogroup C conjugate vaccines in the United Kingdom: reevaluation of correlates of protection. Infect Immun 69: 1568-1573. Link: http://bit.ly/36RFJ67
- Andrews N, Borrow R, Miller E (2003) Validation of serological correlate of protection for meningococcal conjugated vaccine by using efficacy estimates from post licensure surveillance in England. Clin Diagn Lab Immunol 10: 780-786. Link: http://bit.ly/36V8ZJc
- Granoff DM, Feavers IM, Borrow R (2004) Meningococcal vaccines. In Ploktin S, Orenstein WA (eds), Vaccines, 4th ed. Elsevier, Philadelphia, PA 959-988.
- Snape MD, Pollard AJ (2005) Meningococcal polysaccharide-protein conjugate vaccines. Lancet Infect Dis 5: 21-30. Link: http://bit.ly/31mMc7T
- Goldschneider I, Gotschlich EC, Artenstein MS (1969) Human immunity to the meningococcus. I: The role of humoral antibodies. J Exp Med 129: 1307-1326. Link: http://bit.ly/2UmaTAe
- Makela PH, Kayhty H, Weckstrom P, Sivonen A, Renkonen OV (1975) Effect of group-A meningococcal vaccine in army recruits in Filand. Lancet 2: 883-886. Link: http://bit.ly/2RSJFzu
- Peltola H, Makela PH, Kayhty H, Jousimies H, Herva E, et al. (1977) Clinical efficacy of meningococcus group A capsular polysaccharide vaccine in children three months to five years of age. N Engl J Med 297: 686–691. Link: http://bit.ly/31hE5d0
- Holder PK, Maslanka SE, Pais LB, Dykes J, Plikaytis BD, et al. (1995) Assignment of Neisseria meningitidis serogroup A and C class-specific anticapsular antibody concentration to the new standard reference serum CDC1992. Clin Diagn Lab Immunol 2: 132-137. Link: http://bit.ly/3b1X93v
- Vermont C, Dobbelsteen G (2002) Neisseria meningitidis serogroup B: laboratory correlates of protection. FEMS Immunol Med Microbiol 34: 89-96. Link: http://bit.ly/2SaBI7H
- Borrow R, Carlone MG (2001) Serogroup B and C serum bactericidal assays. In Pollard AJ, Maiden MCJ (eds), Meningococcal vaccines – methods and protocols. Humana Press, Totowa, NJ. 289–304. Link: http://bit.ly/2UmbguC
- Borrow R, Aaberge IS, Santos GF, Eudey TL, Oster P, et al. (2005) Interlaboratory standardization of the measurement of serum bactericidal activity by using human complement against meningococcal serogroup B, strain 44/76-SL, before and after vaccination with the Norwegian MenBvac outer membrane vesicle vaccine. Clin Diagn Lab Immunol 12: 970–976. Link: http://bit.ly/2RPVaYm
- Granoff DM, Maslanka SE, Carlone GM, Plikatys BG, Santos GF, et al. (1998) A modified enzyme-linked immunosorbent assay for measurement of antibody responses to meningococcal C polysaccharide that correlate with bactericidal responses. Clin Diagn Lab Immunol 5: 479-485. Link: http://bit.ly/2SaDUfi
- Maslanka SE, Gheesling LL, Libutti DE, Donaldson KB, Harakeh HS, et al. (1997) Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. Clin Diagn Lab Immunol 4: 156-167. Link: http://bit.ly/2uVjK0W
- Maslanka SE, Tappero JW, Plikaytis BD, Brumberg RS, Dykes JK, et al. (1998) Age-dependent Neisseria meningitidis serogroup C class-specific antibody concentrations and bactericidal titers in sera from young children from Montana immunized with a licensed polysaccharide vaccine. Infect and Immun 66: 2453-2459. Link: http://bit.ly/31lqYYn
- Granoff DM, Donnelly JJ (2001) A modified ELISA for measurement of highavidity IgG antibodies to meningococcal serogroup C polysaccharide that correlate with bactericidal titers. Methods Mol Med 66: 305-315. Link: http://bit.ly/2udxGmX
- Zhu BQ, Xu L, Zhou HJ, Shao ZJ (2012) Comparison on the levels of human serum antibody against Neisseria meningitidis serogroup C measured using serum bactericidal assay and ELISA. Zhonghua Liu Xing Bing XueZaZhi 33: 521-524. Link: http://bit.ly/2RSAjDW
- Bertolini DV, Costa LS, van der Heijden IM, Sato HK, Marques HH (2012) Immunogenicity of a meningococcal serogroup C conjugate vaccine in HIV-infected children, adolescents, and young adults. Vaccine 30: 5482–5486. Link: http://bit.ly/2GQ59GQ
- Gheesling LL, Carlone GM, Pais LB, Holder PF, Maslanka SE, et al. (1994) Multicenter comparison of Neisseria meningitidis serogroup C anti-capsular polysaccharide antibody levels measured by a standardized enzyme-linked immunosorbent assay. J Clin Microbiol 32: 1475-1482. Link: http://bit.ly/2UkP6bO
- Holder PK, Maslanka SE, Pais LB, Dykes J, Plikaytis BD, et al. (1995) Assignment of Neisseria meningitidis serogroup A and C class-specific anticapsular antibody concentrations to the new standard reference serum CDC1992. Clin Diagn Lab Immunol 2: 132–137. Link: http://bit.ly/2RTMpwc
- Elie CM, Holder PK, Romero-Steiner S, Carlone GM (2002) Assignment of additional anticapsular antibody concentrations to the Neisseria meningitidis Group A, C, Y, and W-135 meningococcal standard reference serum CDC1992. Clin Diagn Lab Immunol 9: 725-726. Link: http://bit.ly/2vKP2bi
- Borrow R, Richmond P, Kaczmarski EB, Iverson A, Martin SL, et al. (2000) Meningococcal serogroup C-specific IgG antibody responses and serum bactericidal titers in children following vaccination with a meningococcal A/C polysaccharide vaccine. FEMS Immunol Med Microbiol 28: 79-85. Link: http://bit.ly/2ShBXh4
- MacDonald NE, Halperin SA, Law BJ, Danzig LE, Granoff DM (2000) Can meningococcal C conjugate vaccine overcome immune hyporesponsiveness induced by previous administration of plain polysaccharide vaccine? JAMA 283: 1826–1827. Link: http://bit.ly/3900gHd
- Poolman J, Borrow R (2011) Hyporesponsiveness and its clinical implications after vaccination with polysaccharide or glycoconjugate vaccines. Expert Rev Vaccines 10: 307–322. Link: http://bit.ly/3b2Bic2
- Rainwater-Lovett K, Moss WJ (2011) Immunologic basis for revaccination of HIV-infected children receiving HAART. Future Virol 6: 59-71. Link: http://bit.ly/37UIh4N
- Le Guillou H, Le Meur A, Bourdon S, Riou M, Loison J, et al. (2001) Antibody avidity: use for the diagnosis of HIV early infection. Ann Biol Clin 59: 41-47. Link: http://bit.ly/3923A4v
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.



 Save to Mendeley
Save to Mendeley
