Journal of Vaccines and Immunology
Introduction of Stereo Chemical Constraints into β -Amino Acid Residues
Chinnasamy Selvakkumar1*, Karthikeyan Muthusamy2 and Sathishkumar Chinnasamy2
2Department of Bioinformatics, Alagappa University, Karaikudi, Tamil Nadu, India
Cite this as
Selvakkumar C, Muthusamy K,Chinnasamy S (2016) Introduction of Stereo Chemical Constraints into β -Amino Acid Residues. J Vaccines Immun 2(1): 001-003. DOI: 10.17352/jvi.000012Abbreviations
SBT: Seabuckthorn; RVNA: Rabies Virus Neutralizing Antibody; RFFIT: Rapid Fluorescent Focus Inhibition Test; LPS: Lipopolysaccharide; MPL A: Monophosphoryl Lipid A; CTL: Cytotoxic T-lymphocyte; CFTRI: Central Food Technological Research Institute; SC-CO2: Supercritical Carbon Dioxide; SCE: Supercritical Carbon Dioxide Extract; Rb: Inactivated Rabies antigen; QS21: Quillaja saponaria fraction 21; TNF-α: Tumor Necrosis Factor-alpha; IL-1β: InterLeukin-1 beta; CD: Cluster of Differentiation; FBS: Fetal Bovine Serum; PE: Phycoerythrin; FITC: Fluorescein IsoThio Cyanate
Introduction
Over the last 20 years, a large body of work in the literature has focused on the folded structures formed by peptide sequences containing backbone homologated residues. Currently increasing interest in peptide based vaccines for several infectious diseases, and non-infectious diseases. The work of Seebach in Zurich [1] and Gellman in Madison [2], established that oligomers of β amino acid residues can form novel helical structures in solution and in the solid state. Two distinct types of hydrogen bonded helical structures were demonstrated in these studies for oligomeric β peptides. The C12 helix which is an analog of the canonical 3C10 helical structure in “all α” sequences, has the same hydrogen bond directionality (C=Oi…..H-Ni+3). The second helical form, the C14 helix, has the opposite directionality (C=Oi…..H-Ni+4), which is unprecedented in α peptide sequences [3,4]. These reports sparked a flurry of activity on the conformational properties of β peptide oligomers. An early study from Appavu et al. [3-7], had demonstrated that unsubstituted β and γ amino acid residues can be incorporated into oligopeptide helices, without disturbing the overall helical fold [4].
β-amino acid residues
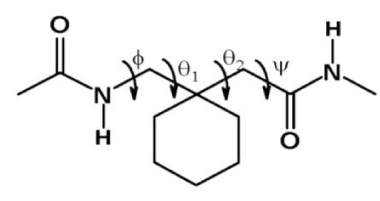
This study suggested that hybrid sequences with expanded hydrogen bonded, rings could indeed be constructed. A very large number of recent studies have greatly expanded our understanding of the conformational properties of substituted β and γ residues, when incorporated into α peptide host sequences [5]. One approach that has been investigated in this laboratory is to examine the role of gem dialkyl substitution on the conformational properties of β and γ residues. The ready availability of the achiral β, β-disubstituted γ amino acid, gabapentin (1-aminomethylcyclohexaneacetic acid, Gpn), has permitted detailed exploration of the structural combinations, (+60°, +60°) and (-60°, -60°) are indicated. (21 structures in (a) and 11 in (b)) (Figure 1).
The presence of gem dialkyl substituents at the central β carbon atom, limits the accessible conformations about the Cβ-Cγ(θ1), and Cβ-Cα(θ2) bonds. A large body of crystallographic evidence has been presented, which suggests that in the case of Gpn θ1, and θ2, are largely restricted to gauche conformations (θ1±60, θ2±60), a property that favors locally folded conformation at this residue. Consequently, a very large number of folded peptides have been characterized, in which diverse hydrogen bonded rings are facilitated by the gabapentin residue [2-7]. Figure 2 provides a summary of the conformational characteristics for the gabapentin residue.
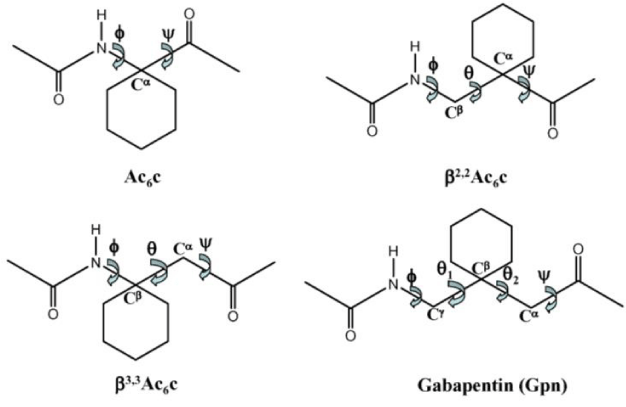
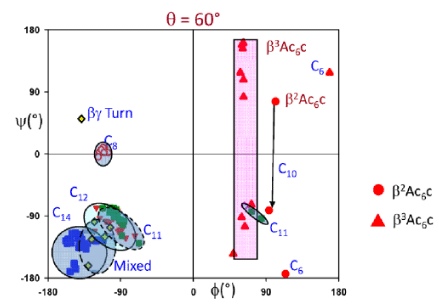
The success in generating folded structures in hybrid peptides containing, the gabapentin residue prompted an examination of the related β-amino acid residues 1-aminomethylcyclohexane carboxylic acid (β2,2Ac6c), and 1-aminocyclohexane acetic acid (β3,3Acc). Figure 3 shows the structures of the four related residues, all of which possess 1,1-disubstituted cyclohexane rings. The parent α amino acid residue 1-aminocyclohexane 1-carboxylic acid (Ac6c) has been conformational characterized in a number of synthetic peptides [8]. The Ac6c residue strongly favors helical conformations, with φ60±30, and ψ30±20. Thus, both β-turn and 3C10/α-helical structures can readily accommodate the Ac6c residue. Two β amino acid homologs may be considered viz β2,2Ac6c, and β3,3Acc. Earlier studies from this laboratory focused on the more readily synthetically accessible residue β3,3Acc.3,4,5,6,7,8 X-ray crystallographic characterization of a number of small peptides containing the β3,3Acc residue revealed that internally hydrogen bonded conformations were rarely observed. The overwhelming majority of the β-amino acid residues (149) adopt gauche conformation (θ=±60°). Out of a total of 210 examples, 61 residues adopt the Trans conformation (φ=-180°). Figure 4 provides a summary of φ,ψ values for all β amino acid residues in which θ values of ±60° have been obtained. Most β3,3Acc amino acid residue fall out outside the region, expected for intramolecularly hydrogen bonded structures, which have been characterized for other β amino acid residues. Only one example of a hydrogen bonded hybrid αβ C11 turn has been observed in Piv-Pro-β3,3Acc-NHMe. These results suggest that the intrinsic conformational preferences of the β3,3Acc residue may not readily facilitate its incorporation into folded, intramolecular hydrogen bonded structures in short peptides. Therefore, an examination of the conformational properties of the isomeric β2,2Ac6c residue was undertaken.
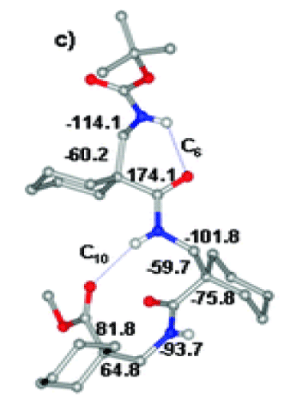
Thus far, relatively few structural reports are available for β2,2Ac6c residue. Figure 5 shows a view of the structure of the peptide Boc-β2,2Ac6c-β2,2Ac6c-β2,2Ac6c-OMe which was already reported at the time these studies undertaken. In this structure the unusual ββ C10 hydrogen bond with reverse directionality and a C6 hydrogen bond were [9,10]. Chapter 5 describes hydrogen bonded C11 turns obtained in αβ hybrid sequences incorporating the β2,2Ac6c residue.
- Seebach D, Gardiner J (2008) Beta-peptidic peptidomimetics. Acc Chem Res 41: 1366-1375 .
- Horne WS, Gellman SH (2008) Foldamers with heterogeneous backbones. Acc Chem Res 41: 1399-1408 .
- Rajagoapl A, Charles BC, Alexey YK, Joshua DS, Frederick JK, et al. (2015) Enhancing the Magnitude of Antibody Responses through Biomaterial Stereochemistry. ACS Biomater Sci Eng 1: 601-609 .
- Rajagopal A, Aravinda S, Raghothama S, Shamala N, Balaram P (2012) Aromatic interactions in model peptide β-hairpins: Ring current effects on proton chemical shifts. Biopolymers 98: 185-194 .
- Rajagopal A, Aravinda S, Raghothama S, Shamala N, Balaram P (2011) Chain length effects on helix-hairpin distribution in short peptides with Aid-DAla and Aib-Aib Segments. Biopolymers 96: 744-756 .
- Raghavender US, Bhaswati B, Indranil S, Rajagopal A, Shamala N, et al. (2011) Entrapement of a Water Wire in a Hydrophobic Peptide Channel with an Aromatic Lining. J Phys Chem B 115: 9236-9243 .
- Krishnayan B, Rajagopal A, Raghothama S, Shamala N, Balaram P (2012) β-Turn Analogues in Model αβ-Hybrid Peptides: Structural Characterization of Peptides containing β2.2Ac6c and β3.3Ac6c Residues. Chem Asian J 7: 1671-1678 .
- Toniolo C, Benedetti E (1991) The polypeptide 3C10-helix. Trends Biochem.Sci 16: 350-353.
- Appella DH, Christianson LA, Karle IL, Powell DR, Gellman SH (1999) Synthesis and Characterization of Trans-2-Aminocyclohexanecarboxylic Acid Oligomers: An Unnatural Helical Secondary Structure and Implications for β-Peptide Tertiary Structure. J Am Chem Soc 121: 6206-6212 .
- Vasudev PG, Ananda K, Chatterjee S, Aravinda S, Shamala N, et al. (2007) Hybrid Peptide Design. Hydrogen Bonded Conformations in Peptides Containing the Stereochemically Constrained γ-Amino Acid Residue, Gabapentin. J Am Chem Soc 129: 4039-4048 .
- Koyfman AY, Rajagopal A, Samantha S, Rudra JS (2015) Self-Assembly of Heterochiral Peptides with Varied Sequence Patterns.
- Rudra JS, Ding Y, Neelakantan H, Chunyong D, Rajagopal A, et al. (2016) Suppression of Cocaine-evoked Hyperactivity by Self adjuvanting and Multivalent Peptide Nanofiber Vaccines. ACS Chem Neurosci.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
