Journal of Vaccines and Immunology
Mild Encephalitis/Encephalopathy with a Reversible Splenial Lesion Associated with Rhinovirus Infection
N Zafer Kurugol1, Sule Gokce2, Cenk Eraslan2, E Ulas Saz3, Sirmen Kizilcan2 and M Ozgur Cogullu4
2Department of Pediatrics, MD, Ege University Medical Faculty, Turkey
3Department of Pediatrics, Associated Professor, Ege University Medical Faculty, Section of Pediatric Emergency Medicine, Turkey
4Department of Pediatrics, Professor Doctor, Ege University Medical Faculty, Division of Pediatric Genetics, Turkey
Cite this as
Kurugol NZ, Gokce S, Eraslan C, Saz EU, Kizilcan S, et al. (2015) Mild Encephalitis/Encephalopathy with a Reversible Splenial Lesion Associated with Rhinovirus Infection. J Vaccines Immun 1(1): 036-038. DOI: 10.17352/jvi.000008We report a 7-year-old patient with mild encephalopathy with a reversible splenial lesion (MERS) presenting with recurrent delirious behavior, hallucinations and seizures following common cold. Cranial MRI showed high signal intensity in the splenium of the corpus callosum. Rhinovirus was detected in the nasopharyngeal swab by multiplex PCR. Other respiratory viruses were not detected. Microbiologic tests for Epstein-Barr virus, herpes simplex virus, varicella-zoster virus, cytomegalovirus, measles, mumps, rubella and Mycoplasma pneumoniae were also negative. This is the first reported case of MERS associated with rhinovirus infection.
Introduction
Mild encephalitis/encephalopathy with a reversible splenial lesion (MERS) is a clinoco-radiological entity first described by Tada et al. [1]. The clinical manifestations consist of relatively mild neurologic symptoms, most commonly delirious behavior, consciousness disturbance and seizures. Magnetic resonance imaging (MRI) typically shows reversible lesions with reduced diffusion in the splenium of the corpus callosum (SCC), sometimes associated with symmetrical white matter lesions [2]. The clinico-radiological entity has been mainly described in patients of South-East-Asian origin. There have been only few case reports from Europe or North America [3,4]. Previous studies have suggested that MERS is mostly associated with influenza virus, mumps virus, rotavirus and adenovirus infections [2,5]. It has also been reported to be associated with other infectious diseases such as parainfluenza virus, measles, human herpesvirus-6, varicella-zoster virus, parvovirus B19, Epstein-Barr virus, streptococcus, Escherichia coli, Salmonella enteritidis, Mycoplasma pneumonia and Legionella pneumophila. Similar findings have been described in some noninfectious conditions such as antiepileptic medication, Kawasaki disease and hypoglycemia. Familial cases have also been published. In this report, we present a case of MERS associated with rhinovirus infection. To our knowledge, this is the first description of MERS in association with rhinovirus infection.
Case Report
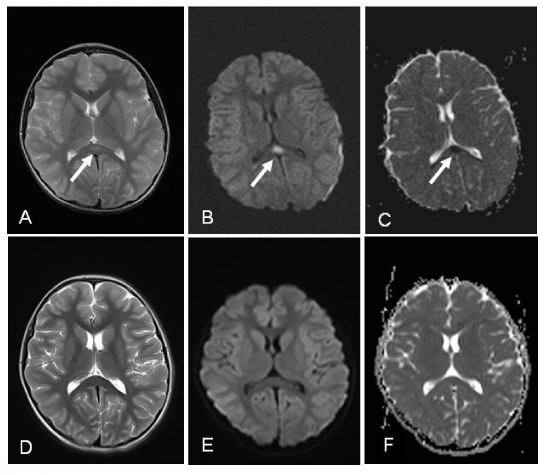
A previously healthy 7-year-old girl was admitted to our emergency department with recurrent delirious behavior, seizures, paresthesia in the upper and lower extremities and refusing to walk. Three days before admission, she developed sneezing, cough, rhinorrhea and nasal congestion. There was no family history or past history of neurological disorders. She was not on any medication (e.g., antiepileptic). On admission, she was conscious, but suffered from delirious behavior, hallucinations and refused to walk. Her body temperature was elevated, at 38.2 °C. Clinical examination revealed no focal neurologic signs, but signs of upper respiratory tract infection were noted. Routine blood counts and biochemical investigations, including serum sodium level, were normal. Cerebrospinal fluid (CSF) examinations showed normal cell counts, and protein and glucose levels, and CSF cultures were bacteriologically sterile. Polymerase chain reaction (PCR) assays of CSF for herpes simplex virus 1 and 2, influenza virus, adenovirus, enterovirus, cytomegalovirus, human herpesvirus-6, Epstein-Barr virus and varicella zoster virus were all negative. Electroencephalography was normal. Cranial MRI showed a high intensity signal in the splenium of the corpus callosum on T2- weighted and diffusion weighted images (Figure 1A, 1B). A low apparent diffusion coefficient (ADC) was noted in the same area (Figure 1C).
Rhinovirus was detected in a nasopharyngeal swab specimen by multiplex PCR. PCR products were detected by automated polyacrylamide gel electrophoresis using Screen Tape multiple detection system. Specimens which were positive for viral nucleic acids have been further studied by using specific DPO primers, FluA ACE Subtyping and RV15 Screening (Seegene, South Korea) kits. Rhinovirus types A and B, four influenza-A virus subtype human H1 (hH1), human H3 (hH3), swine H1 (sH1), avian H5 (aH5)] and 11 other respiratory viruses [Adenovirus, parainfluenza virus (PIV) types 1-4, human bocavirus (HBoV), human metapneumovirus (HMPV), human coronaviruses (HCoV) OC43, 229E/NL63] were investigated with those tests. While multiplex PCR for other respiratory viruses were negative, rhinovirus was positive. Microbiologic tests for Epstein-Barr virus, herpes simplex virus, varicella-zoster virus, cytomegalovirus, measles, mumps, rubella and Mycoplasma pneumoniae were also negative. Empirical treatment with acyclovir and oseltamivir was administered until the result of laboratory investigations for herpes and influenza virus proved to be negative. However, the patient’s hallucinations and delirious behavior persisted, and seizures continued to occur several times daily. She also became drowsy and confused. Follow up MRI revealed similar abnormal signal changes in the same area as observed in the previous MRI. Therefore, intravenous pulse methylprednisolone therapy (30 mg/kg/dose daily) was started on day 10. After pulse steroid therapy, the patient improved rapidly, and her delirious behavior and hallucinations completely disappeared within 24 hours. The SCC lesions on T2- weighted (Figure 1D), diffusion weighted images (Figure 1E) and apparent diffusion coefficient map (Figure 1F) completely disappeared.
Discussion
We have described a case of a previously healthy child who developed MERS secondary to rhinovirus infection. Human rhinoviruses (HRVs) are the most common human respiratory pathogens and are responsible for most upper respiratory infections (e.g., common cold). They may also cause severe lower respiratory tract infections, including pneumonia and bronchiolitis [6]. Fatal HRVs bronchiolitis have recently been reported in Vietnam [7]. Additionally, HRVs are known as a major pathogen for asthma exacerbations. Nonrespiratory symptoms, however, are very rare [8]. The association of MERS with rhinovirus infection has not been previously reported in the literature.
Several studies have reported that MERS is associated with a good prognosis [3,9,10]. A recent surveillance study conducted in Japan suggested that the vast majority of patients with MERS (138 of 153 cases, 90.2%) completely recovered without any sequelae [5]. In the remaining patients (11 cases, 7.1%), mild or moderate neurologic sequelae were observed. No serious permanent sequelae or death were reported. Clinical recovery is usually achieved within a few weeks, even without any treatment [10]. In clinical practice, however, pulse methylprednisolone, intravenous immunoglobulin, acyclovir, oseltamivir, antibiotics and anti-epileptic drugs have been used in the treatment of patients with MERS [2,3,10,13]. Our patient was also treated with oseltamivir because MERS has mainly been associated with influenza A and B virus infection. Despite the anti-viral therapy, however, her neurologic symptoms such as delirious behavior, hallucinations and seizures increased daily. The patient had also become drowsy and confused. Intravenous pulse methylprednisolone therapy was therefore given on day 10, and a rapid and complete clinical recovery was achieved within 24 hours. Similar cases of MERS showing rapid recovery following steroid pulse therapy have also been previously reported in the literature [11,14,15]. However, since many patients with MERS have recovered without the administration of corticosteroid therapy, it is difficult to conclude the necessity of steroid pulse therapy for MERS.
- Tada H1, Takanashi J, Barkovich AJ, Oba H, Maeda M, et al. (2004) Clinically mild encephalitis/encephalopathy with a reversible splenial lesion. Neurology 63:1854-1862 .
- Takanashi J (2009) Two newly proposed infectious encephalitis/encephalopathy syndromes. Brain Dev 31: 521–529 .
- Abenhaim Halpern L, Agyeman P, Steinlin M, El-Koussy M, Grunt S (2013) Mild encephalopathy with splenial lesion and parainfluenza virus infection. Pediatr Neurol 48: 252-254 .
- Melenotte C, Craighero F, Girard N, Brouqui P, Botelho-Nevers E (2013) Measles encephalitis the return: mild encephalitis with reversible splenial lesion. Int J Infect Dis 72-73 .
- Hoshino A, Saitoh M, Oka A, Okumura A, Kubota M, et al. (2012) Epidemiology of acute encephalopathy in Japan, with emphasis on the association of viruses and syndromes. Brain Dev 34: 337-343.
- Ruuskanen O, Waris M, Ramilo O (2013) New aspects on human rhinovirus infections. Pediatr Infect Dis J 32: 553-555.
- Hai le T, Bich VT, Ngai le K, Diep NT, Phuc PH, et al. (2012) Fatal respiratory infections associated with rhinovirus outbreak, Vietnam. Emerg Infect Dis 18: 1886-1888.
- Spencer MJ, Cherry JD, Adams FH, Byatt PH (1975) Letter: Supraventricular tachycardia in an infant associated with a rhinoviral infection. J Pediatr 86: 811-812 .
- Sharma B, Handa R, Nagpal K, Prakash S, Panagariya A (2014) Transient elevation of cerebrospinal fluid protein in a patient of mild encephalitis with reversible lesion in the splenium: a case report. Malays J Med Sci 21: 94-97 .
- Takanashi J, Imamura A, Hayakawa F, Terada H (2010) Differences in the time course of splenial and white matter lesions in clinically mild encephalitis/encephalopathy with a reversible splenial lesion (MERS). J Neurol Sci 292: 24-27 .
- Hara M, Mizuochi T, Kawano G, Koike T, Shibuya I, et al. (2011) A case of clinically mild encephalitis with a reversible splenial lesion (MERS) after mumps vaccination. Brain Dev 33: 842-844 .
- Hibino M, Hibi M, Akazawa K, Hikino K, Oe M (2011) A case of Legionnaires' pneumonia accompanied by clinically mild encephalitis/encephalopathy with a reversible splenial lesion (MERS) with transient altered mental status and cerebellar symptoms, which responded to treatment by antibiotics and corticosteroid. Nihon Kokyuki Gakkai Zasshi 49: 651-657 .
- Osuka S, Imai H, Ishikawa E, Matsushita A, Yamamoto T, et al. (2010) Mild encephalitis/encephalopathy with a reversible splenial lesion: evaluation by diffusiontensor imaging. Two case reports. Neurol Med Chir 50: 1118-1122 .
- Hatanaka M, Kashiwagi M, Tanabe T, Nakahara H, Ohta K, et al. (2015) Overlapping MERS and mild AESD caused by HHV-6 infection. Brain Dev 37: 334-338.
- Imamura T, Takanashi J, Yasugi J, Terada H, Nishimura A (2010) Sisters with clinically mild encephalopathy with a reversible splenial lesion (MERS)-like features; Familial MERS? J Neurol Sci 290: 153-156 .
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
