Global Journal of Infectious Diseases and Clinical Research
Sero-prevalence of Hepatitis B and C virus and High Risk of Hepatotoxicity among TB/HIV Positive and HIV Negative Population in Western Cameroon
Leonard Fonkeng Sama1, Olive Ismae1 Nganou Djinou1, Elvis Chongsi Wam1, Roland Bamou2, Innocent Mbulli Ali1,3, Michel Noubom4-6 and Christopher Bonglavnyuy Tume1*
2Laboratory of Applied Biology and Ecology, Department of Animal Biology, University of Dschang, P.O. Box 67, Dschang, Cameroon
3Laboratory for Public Health Research Biotechnologies, The Biotechnology Centre, University of Yaoundé 1, P.O. Box 8094, Yaoundé, Cameroon
4Department of Biomedical Science, University of Dschang, Cameroon P.O. Box 67 Dschang
5Centre Médical d’Arrondissement [CMA] de Baleng Bafoussam
6Centre de Diagnostic et de Traitement de la Tuberculose, P.O. Box 01 Bafoussam
Cite this as
Sama LF, Nganou Djinou OI, Wam EC, Bamou R, Ali IM, et al. (2017) Sero-prevalence of Hepatitis B and C virus and High Risk of Hepatotoxicity among TB/HIV Positive and HIV Negative Population in Western Cameroon. Glob J Infect Dis Clin Res 3(1): 001-006. DOI: 10.17352/2455-5363.000011Background: Hepatitis and HIV are the most common co-infections in tuberculosis (TB) patients and may have an effect on the liver enzymes in these co-infected TB patients. This study aimed to determine the prevalence of Hepatitis B, C and HIV in patients infected with TB in Western Cameroon and assess the effects of co-infection on their liver function.
Materials and Methods: All TB infected patients referred to the Tuberculosis Research centres, from November 2014 to July 2015, and who gave their consent were screened for Hepatitis B surface antigen (HBsAg) & Hepatitis C virus (VHC) antibodies using enzyme linked immunoabsorbent assay (ELISA). HIV infection was confirmed using a combination of two rapid tests, namely, Combaids and Tridot. All HIV positive patients were on antiretroviral therapy during the study period. The data was entered and analysed using statistical Package for social sciences 21 (SPSS– 21), and the means and proportions were calculated.
Results: Of the 189 tuberculosis patients recruited in this study, HBsAg were detected in 24 (12.7%), anti-VHC antibodies in 8 (4.23%), HIV antibodies in 62 (32.8.0%), HBsAg and anti-VHC antibodies in 1 (0.53%), HBsAg and HIV antibodies in 9 (4.9%), and anti-VHC and HIV antibodies in 2 (1.1%). Estimation of liver enzymes in all co-infected and TB patients showed that a substantial proportion of our patients had normal ALP and GGT levels whereas a substantial proportion of our patients had abnormal levels of ALT and AST with patients having up to two to three-fold. All the study groups had higher baseline AST and ALT values with VHB co-infected groups having the biggest mean values.
Conclusions: The prevalence of hepatitis B and C coinfection was fairly high in this largely heterosexual population supporting the use of more careful screening methods for these viruses in tuberculosis persons in these regions. High levels of transaminases were found in our study population suggesting that all TB patient should be screened for VHB, VHC and HIV infections, then monitored carefully following the initiation of therapy.
Abbreviations
ALP: Alkaline Phosphatase; ALT: Alanine aminotransferase; AST: Aspartate transaminase; CMA: Medicalised Health Centre; GGT: gamma-glutamyltransferase; RHB: Regional Hospital Bamenda; TB: Tuberculosis; VHB: Viral Hepatitis B; VHC: Viral Hepatitis C; WHO: World Health Organisation
Introduction
Viral hepatitis, particularly hepatitis B virus (VHB) and hepatitis C virus (VHC) infections, are global public health concerns because they are the leading cause of chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma [1]. Hepatitis B (VHB) Virus is a major public health problem worldwide with about one third of the world’s population infected, amongst which 400 million have a chronic infection and 350 million remaining asymptomatic carriers [2-4]. Chronic VHC infection also affects approximately 200 million individuals, that is, 2.5% of the world’s population [5]. Hepatitis B and C show great diversity in their prevalence in different parts of the world. Global and region-specific estimates of VHB and VHC prevalence vary greatly, but the highest prevalence (15–20%) has been reported in Egypt [6,7].
Tuberculosis remains a leading health problem in both developing and developed countries [5]. The World Health Organization (WHO) estimated that there were 9.6 million new cases of TB globally in 2014 and 1.5 million deaths [8]. This is increased by HIV which is recognized alongside as a leading cause of death worldwide. In 2013, 1.5 [1.4–1.7] million people died from HIV-related causes globally. In the same year there were approximately 35.0 [33.2–37.2] million people living with HIV with 2.1 [1.9–2.4] million people becoming newly infected globally. Sub-Saharan Africa is the most affected region, with 24.7 [23.5-26.1] million people living with HIV that accounts for almost 70% of the global total of new HIV infections [9].
According to a recent report by the World Health Organization, Cameroon is a relatively high-TB-burden country in Central Africa with an estimated prevalence of all form includ HIV was 319 [153-344]/100.000 and the incidence rate with HIV case was 238 [117-283]/100.000 /100,000 resident and a case-detection rate of 60% [10].
Globally, the prevalence of VHB and VHC infections among patients with TB has not been extensively investigated, and very limited data on the rate of VHB and VHC co-infection among patients with TB exists [11]. Hepatotoxicity is the major adverse effect of three of the first line anti-TB agents: isoniazid, rifampin, and pyrazinamide [12]. Chronic liver disease raises a risk of hepatotoxicity during anti-tuberculosis treatment, which is three to five times more than that in TB patients who do not have viral infection [13]. Similarly, fourteen fold increase in the risk of anti-TB hepatotoxicity has been reported in HIV and VHC co-infected patients [14]. Patients with increased susceptibility to the hepatotoxic effects of first-line treatment regimens represent special populations and need to be identified prior to therapy initiation and monitored more carefully. Although three of the first-line drugs, rifampin, pyrazinamide, and isoniazid are known to be hepatotoxic [15-20], the patient characteristics that confer greater risk of treatment-associated liver injury are poorly understood. Older age, concurrent or chronic alcohol use, hepatitis C, hepatitis B, and HIV virus infection have been found to increase the risk of drug induce hepatotoxicity (DIH) [21-23]. Until definitive studies are conducted, caution suggests that patient populations should be screened for the above-mentioned characteristics and monitored carefully following the initiation of therapy [24]. Therefore, this study was conducted to address the prevalence of VHB, VHC and HIV infections in patients with TB and its impact on the incidence of anti-tuberculosis therapy induced hepatotoxicity.
Materials and Methods
Study area
This study was carried out at the TB unit of the Regional Hospital Bamenda [RHB] situated in the Nord-West Region of Cameroon and at the TB unit of the “Centre Médical d’Arrondissement (CMA) de Baleng Bafoussam” located in the West Region of Cameroon. The two hospitals have dedicated adult pulmonology wards primarily for admission of TB patients during the intensive phase of treatment.
Enrolled in this study were patients who were admitted on the pulmonology wards during the study period and who had a confirmed microscopic diagnosis of pulmonary TB. All the patients who refused informed consent and minors who provided assent but whose guardians/legal representatives refused to consent were excluded from the study.
This was a transversal cross sectional study in which patients with a microscopically confirmed diagnosis of pulmonary TB admitted on the TB wards of the Regional Hospital Bamenda (RHB) and CMA Baleng Bafoussam were consecutively recruited during the study period from November 2014 to July 2015. Collectively, these two study centres include more than 80% of the state’s incident TB cases and have dedicated adult pulmonology wards primarily for admission of TB patients during the intensive phase of treatment.
Ethical considerations
Ethical clearance for this study was obtained from the Ethics Review and Consultancy Committee of the Cameroon Bioethics Initiative [CAMBIN] under the reference number CBI/294/ERCC/CAMBIN on the 16th of September 2014. An authorisation to collect and analyse blood samples was also obtained from the Regional Delegation of Public Health Bamenda and the Baleng Medicalised Centre in Bafoussam. All participants were fully informed of the study goals, procedures, potential harm and benefits, cost as well as the finality of the study. They willingly provided informed consent either by signing or placing their thumbprint on the consent form after being satisfied with responses to all questions asked the investigator. Information was provided in English, French or interpreted in the local dialect by a hospital volunteer independent of the study team.
Sample size
In the present study, a convenient sample of 189 sputum positive pulmonary TB patients who visited the health facilities and who provided consent prior to the onset of any study procedure were included. These sample size was designed according to the prevalence of the co-infection between these two diseases which is 1% to 18%.
Laboratory investigation and specimen collection
All case records were investigated for clinical history (including history of intravenous drug use, other risk behaviours), physical examination findings and laboratory investigation results.
Venous blood samples of about 2-5 ml were collected aseptically from each participant by the assigned laboratory technician. These blood specimens were appropriately transported to the Laboratory for further processing to obtain sera which was then used for testing VHC antibodies, VHB antigens, HIV antibodies and the measurement of liver enzymes; alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) and gammaglutamyltransferase.
All serum samples were screened for hepatitis B surface antigen (HBsAg) by ELISA (Hepalisa, J. Mitra & Co. Ltd/Biotech INC, India), and for anti-VHC antibodies by ELISA (Micro Lisa, J. Mitra& Co. Ltd/Biotech INC, India) and the cut off values were calculated as per the manufacturer’s instructions manual. Any value above the cut off was taken as positive for HBsAg/ VHC. Positive results on ELISA were confirmed by a second test (Hepacard for Hepatitis B and RIBA assay for hepatitis C from Biotech INC, India). HIV infection was confirmed using a combination of two rapid tests namely Combaids (Span Diagnostics, India) and Tridot (J. Mitra &Co.Ltd/Biotech INC, India) and one ELISA (Lab systems, U.K.) as per the guidelines of National AIDS control organization (NACO). Pre-test counselling was done and informed consent obtained from the patient before performing an HIV test. Liver function tests (Aspartate aminotransferase (AST), Alanine aminotransferase (ALT) Alkaline phosphatase (ALP) and gammaglutamyltransferase (GGT)) by clinical auto-analyser, (Model: ACE, Schiapparelli Biosystems. INC, Netherlands).
Tuberculosis was diagnosed on the basis of sputum smear, culture results for M. tuberculosis with clinical and radiographic evidence of tuberculosis.
Statistical Analysis
The data was entered and analysed using statistical Package for social sciences 21 (SPSS– 21), the means and proportions were calculated.
Results
Socio-demographic characteristics of study subjects
A total of 189 TB patients were included in the study. Of the total TB patients, 120 (63.49%) were male while 69 (36.51%) of them were female with male to female ratio of 1.74:1. The mean age of the respondents was 39.05 ± 14.305 years with minimal being 12 years and the maximal 82 years. Concerning the marital and education status of the study subjects, 109 (57.67%) were married, 76 (40.21%) were single and 4 (2.12%) were widowed whereas 91 (48.15%) had primary grade, 86(45.50%) had secondary grade, 8 (4.23%) had university degree and 4 (2.12%) had never gone to school. Concerning the serological status of the study subjects, 116 (61.4%) were HIV negative and 73 (38.6%) were HIV positive. The baseline characteristics of these socio-demographic characteristics of the patients is shown in table 1.
Prevalence of VHB, VHC and HIV among the TB patients
Using ELISA to test for hepatitis B surface antigen (HBsAg) (Hepalisa, J. Mitra & Co. Ltd/Biotech INC, India), anti-VHC antibodies (Micro Lisa, J. Mitra & Co. Ltd/Biotech INC, India) and Human immunodeficiency virus chart documentation of serologic testing and the cut off values considered as positive, HBsAg were detected in 24 (12.7%), anti-VHC antibodies in 8 (4.23%), HIV antibodies in 62 (32.8.0%), HBsAg and anti-VHC antibodies in 1 (0.53%), HBsAg and HIV antibodies in 9 (4.9%) and anti-VHC and HIV antibodies in 2 (1.1%) patients as shown in table 2.
There were more males, 20 (83.3%) positive to VHB compared to females, 4 (16.7%) (P=0.029). In a similar way, there were more males 6 (75.0%) positive to VHC compared to females 2 (25.0%), although the difference was not significant (P=0.13). Moreover, there were more HIV positive females 38 (52.1%) compared to males 35 (47.9%) (P=0.001). The patients aged between [22-35], 10 (41.4%) and [36-55], 10 (41.4%) were more positive to VHB (P=0.01) whereas patients aged >55 6 (75.0) were more positive to VHC compared to patients of the other groups although the difference was not significant (P=0.3128). The age groups [22-35], 30 (41.1%) and [36-55], 34 (46.6%) were the most infected with HIV although the difference was not significant (P=0.197). There were more patients with primary level of education, 4 (50.00%) positive to VHB compared to other groups although the difference was not significant (P=0.098). Similarly, patients with primary level of education, 38 (52.1%) were the most positive to HIV infection compared to patients of the other groups although the difference was not significant (P=0.708). In addition, patients with secondary level of education, 15 (62.1%) were the most positive to VHB compared to the other groups although the difference was not significant (P=0.255). The married and unmarried participants in this study were highly infected with VHB compared to the widowed although the difference was not significant (P=0.48). However, married participants were the most positive to HIV infection compared to the other groups although the difference was not significant (P=0.504). Similarly, married participants were the most positive to VHC compared to the other groups although the difference was not significant as shown in table 3.
The baseline values of liver enzymes of all the infected and co-infected patients are shown in table 4.
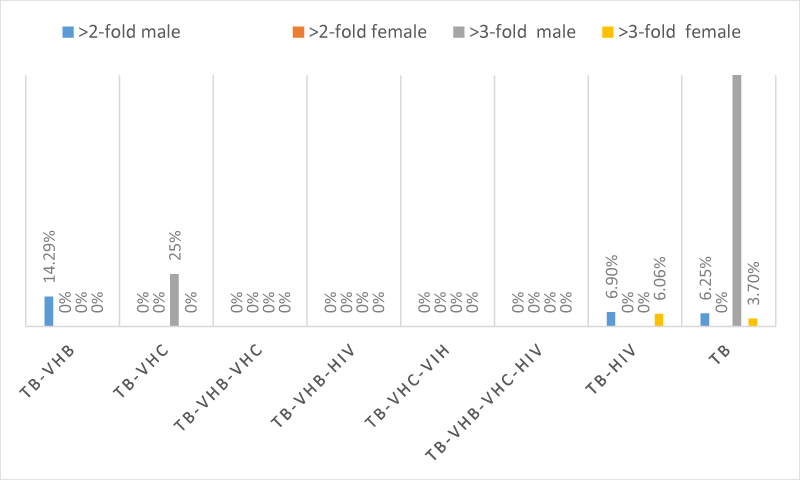
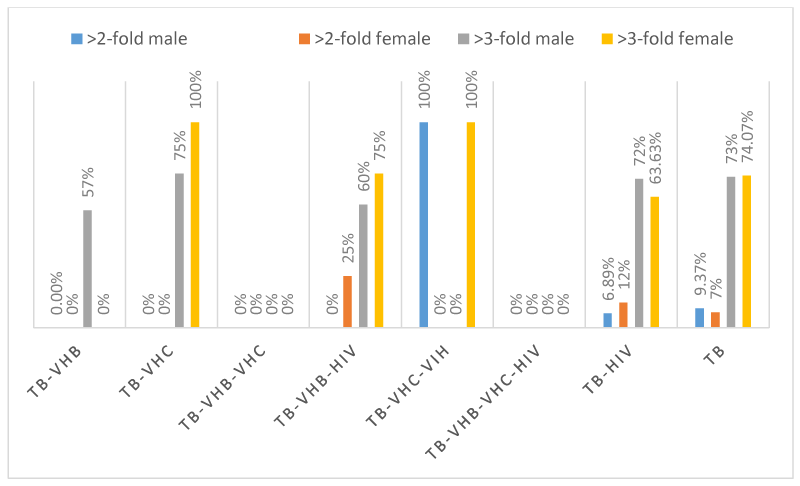
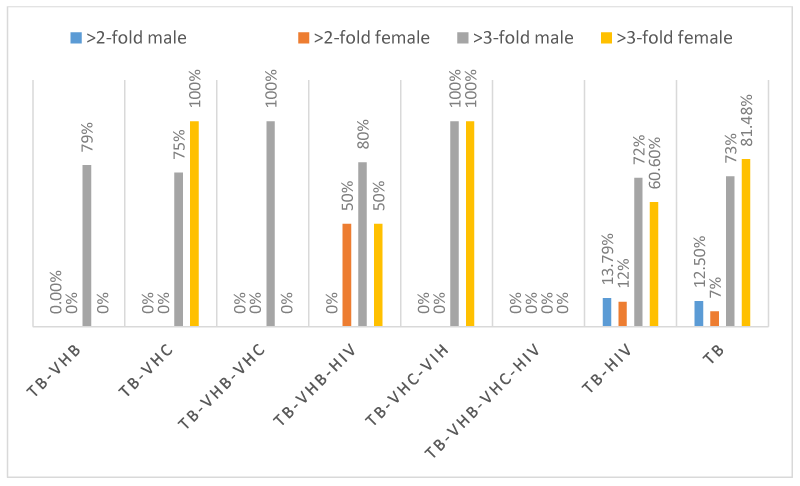
The evaluation of hepatic enzymes (GGT, ALT, AST, and ALP) in the 189 patients showed that, only 2 patients with TB-VHB, 2 patients with TB-HIV and 4 patients had more than two-fold elevations in GGT, whereas 2 patients with TB-HIV and 5 patients with TB had more than three-fold elevations in GGT. One (1) patient with TB-VHB-HIV, 1 patient with TB-VHC-HIIV, 6 patients with TB-HIV and 8 patients had more than two-fold elevations in ALT whereas 8 patients with TB-VHB, 4 patients with TB-VHC, 6 patients with TB-VHB-HIV, 1 patient with TB-VHC-HIV, 42 patients with TB-HIV and 67 patients with TB had more than three-fold elevations in ALT. Four (04) patients with TB-HIV and 3 patients with TB had more than two-fold elevations in ALP whereas 1 patient with TB-VHB, 1 patient with TB-VHB-HIV, 5 patients with TB-HIV and 3 patients with TB had more than three-fold elevations in ALP. Two (02) patients with TB-VHB-HIV, 8 patients with TB-HIV and 10 patients with TB had more than two-fold elevations in AST, whereas 11 patients with TB-VHB, 4 patients with TB-VHC, 1 patients with TB-VHB-VHC, 6 patients with TB-VHB-HIV, 2 patients with TB-VHC-HIV, 41 patients with TB-HIV and 69 patients with TB. The schematic repartition of patients having enzymatic alterations and according to the sex are represented in Figures 1-4 depending on the type of enzyme. It is seen from those figures that there were patients having more than two to three-fold elevation of AST and ALT.
Discussion
To our knowledge this is the first report conducted in Western Cameroon to investigate prevalence of VHB and VHC infections among TB/HIV positive and TB/HIV negative patients and the liver profile. The overall prevalence of VHB, VHC and HIV infections in the current study was 12.7%, 4.23% and 32.8.0% respectively. This prevalence of VHB (12.7%) is in agreement with the fact that Cameroon like other sub-Saharan African countries is a hyper-endemic area for hepatitis B infection (prevalence 8-20%) [25,26]. The seropositivity of VHB infection, (12.7%) found in this study was relatively higher than the 9.5% obtained in the study conducted by Nail et al., in Khartoum among TB patients, 9.4% obtained by Khalid and Bahaeldin in sudanese patients with HIV/TB co-infection, 8.92% obtained by Mengesha et al., in Ethiopia, and also higher than that reported from other studies carried out, in Thailand (9%), Georgia (4.3%), Pakistan (5.5%), Kurdistan (1.8%), and India (2.96%) [1,27-30,36-45]. However the 12.7% obtained from this study was less than the 15.3% reported by Abdallah et al., in Eastern Sudan and 18.4% reported in London by Nooredinvand et al. [31,36]. The present prevalence obtain in the present study was almost the same as the national prevalence which is 12.14% [32].The proportion of VHB/TB co-infection was found to be higher in males (10.58%) than females (2.12%). This finding is similar to other reports [27,33,34]. High rate of VHB infection among male gender in this setting might be related to the gender exposure difference between males and females. Interestingly, a male preponderance was documented recently in another study conducted among general population in Eastern Sudan [35]. The high frequency of VHB infection observed in this study may be explained by the higher national prevalence but also lack of adherence to universal infection control measures including vaccination.
The prevalence of VHC infection in the present study was 4.23% which is higher than that reported by Nail et al. (3.5%), Abdallah et al., in Eastern Sudan (1%), and Khalid and Bahaeldin (1.9%). This was also higher than that from other reports carried out, in London (2.6%) by Nooredinvand et al., and 0.9% in Kurdistan by Muayad et al. [1, 27, 31, 36, 43]. The prevalence of VHC infection among general population was reported as 4.3%-29.7% [37]. However, it was lower than that obtained from previous studies done by Reis NR et al. (7.5%) in Brazil, Richards et al. (22%) in Georgia, Akhtar et al. (9.1%) in pakistan, Agha et al. (17.02%) in Egypt, Khalili et al. (27.45%) in Soudan, and Mengesha et al. (7.14%) in Ethiopia, [30,38-41,43,44]. The proportion of VHC/TB co-infection was found to be higher in males (3.17%) than females (1.06%). High rate of VHC infection among male gender in this setting might be related to the gender exposure difference between males and females.
The difference between the prevalence of VHB and VHC infections in the current study and other results may be attributed to use of different diagnostic techniques such as PCR, ELISA, and ICT as reported by Akhtar et al. [30]. Also the sample size may be responsible as recently reported by Abdallah et al., in the prevalence of VHB and VHC among healthy blood donors as 4, 3% and 3.1% respectively [42].
Estimation of Alanine aminotransferase (ALT), Aspartate transaminase (AST), Alkaline Phosphatase (ALP) and gamma-glutamyltransferase (GGT) are an inexpensive and invasive means of assessing liver disease as it reflects the activity of hepatotropic viruses and status of liver before and during therapy with various hepatotoxic drugs. A substantial proportion of our patients had normal ALP and GGT levels whereas a substantial proportion of our patients had abnormal levels of ALT and AST with patients having up to two to three-fold. All the study groups had higher baseline AST and ALT values with VHB co-infected groups having the biggest mean values. 68.34% male and 66.67% of female participants had more than three-fold ALT while 73.34% of male and 66.67% of female participants had more than three-fold AST. This slight difference may be due to hormonal differences between males and females but could also be due to the proportion of males and females in the present study where males were more represented.
In the present study an increase in hepatic transaminase values to more than 2 times the upper limit of normal (>50 IU) occurred in 57.14% of TB-VHB, 20% of TB-VHC, 77.78% of TB-VHB-IHV, 100% of TB-VHC-HIV, 77.42% of TB-HIV and 82.42% of TB for ALT and 78% of TB-VHB, 80% of TB-VHC, 88.89% of TB-VHB-IHV, 100% of TB-VHC-HIV, 100% of TB-VHB-VHC, 79.03 of TB-HIV and 86.81% of TB for AST.
Comparing the results obtained in our study to those from previous studies, we observed that the high levels of transaminases are comparable to those obtained by Subir et al. 2013 [46], but differ to those obtained by Padmapriyadarsini et al. (2006) where substantial proportions of patients had normal ALT and AST levels before treatment [47].
The high levels of transaminases obtained in our study could be explained by the fact that a good number of our study participants were HIV patients (38.6%) who have been on ARV treatment for a long time. It has been shown that HIV attacks the liver cells directly [48], causing cell death and the release of the cellular contents into the surrounding medium, of which the enzymes constitute 20% [49]. This may be responsible for the increase in the serum liver enzymes in the infected patients. As a support to our findings, various studies have shown a significant increase in the serum AST, ALT and ALK-P activities in the ART naïve [50]. Moreover the values are more in the cases with tuberculosis. This may be due to the degeneration of the connective tissue of the liver [51] and it may also be the result of the hepatobiliary obstruction that had occurred in the subjects. This high levels of transaminases may also be due to the fact that, in our society, access to treatment is still difficult due to poverty and the fact that the majority of the population hardly visit the hospital for appropriate treatment unless their health is critical. They always concentrate on taking over-the-counter drugs which in general are drugs from unknown origin, not well preserved and most of the time are expired drugs that can lead to severe hepatotoxicity and cirrhosis as in the case of our patients.
It has been reported that VHB and VHC infections are independently and significantly associated with incidents of hepatotoxicity [52,53] but also HIV-Antiretroviral Therapy [54]. Thus co-infection of tuberculosis, HIV, VHB and VHC increase the risk of hepatotoxicity particularly during treatment of tuberculosis. Therefore, it is important to identify them so as to reduce morbidity and delay mortality.
One of the limitations of this study was that the risk factors and social behaviours for VHB and VHC among TB patients were not assessed but also the sero-prevalence of VHB and VHC were not confirmed by polymerase chain reaction (PCR). So, further study is needed to determine the risk factors of VHB and VHC among tuberculosis patients.
Conclusion
This study documents high prevalence of VHB and VHC infections among TB infected patients; suggesting more careful screening for these viruses in TB positive persons. Further, we have shown a high level of transaminases in our study population. Therefore it should be mandatory to screen every TB patient for VHB and VHC and a careful follow up should be done during TB treatment.
We wish to thank all the participants who sacrificed their time and donated their blood for this study. We also thank particularly the Director, and Nurses of Regional Hospital Bamenda and “Centre Médical d’Arrondissement de Bafoussam” for their contributions and assistance during sample collection and analysis.
Authors’ contributions
CBT, and LFS conceived the study. LFS and OIND carried out sample analysis and data collection. IMA and RB participated in analysis of the samples, data management and statistics. MN supervised the field study. LFS, OIND and ECW drafted the manuscript. All authors reviewed the manuscript and approved the final version prior to submission.
Authors’ information
1. LFS is a PhD student in the Department of Biochemistry of the University of Dschang, Cameroon.
2. Olive Ismael Nganou Djinou is a PhD student in the Department of Biochemistry of the University of Dschang, Cameroon.
3. ECW is a PhD student in the Department of Biochemistry of the University of Dschang, Cameroon.
4. RB is a PhD student in the Department of Animal Biology of the University of Dschang, Cameroon.
5. IMA is a Lecturer in the Department of Biochemistry of the University of Dschang and a Researcher in the Laboratory for Public Health Research Biotechnologies at the Biotechnology Centre, University of Yaounde 1 Cameroon.
6. MN is a medical doctor and lecturer in the Department of Biomedical Sciences in the University of Dschang, Cameroon.
7. CBT is an Associate Professor of Immunology and Molecular Biology in the Department of Biochemistry of the University of Dschang, Cameroon.
- Muayad AM, Safer MH, Abid MHASUM (20160029 Low prevalence of hepatitis B and C among tuberculosis patients in Duhok Province, Kurdistan: Are HBsAg and anti-VHC prerequisite screening parameters in tuberculosis control program? Int J Mycobacteriol 5: 313-317. Link: https://goo.gl/9tuARu
- Lok AS, McMahon BJ (2007) Chronic hepatitis B, Hepatology. 45: 507-539. Link: https://goo.gl/X2PyCL
- Al-Jabir AA, Al-Adawi S, Al-Abri JH, Al-Dhahry SH (2004) Awareness of hepatitis B virus among undergraduate medical and non-medical students. Saudi Med J 25: 484-487. Link: https://goo.gl/c4yicF
- Anjum QH, Siddiqui Y, Ahmed SR, Usman YR (2005) Knowledge of Students regarding Hepatitis and HIV/AIDS of a Private Medical University in Karachi. J Pak Med Assoc 55: 285-288. Link: https://goo.gl/lUAGuB
- European Association for Study of Liver (2014) Clinical practice guidelines: management of hepatitis C virus infection. J Hepatol 60: 392-420. Link: https://goo.gl/gzsEjJ
- Hajarizadeh J, Grebely G, Dore (2013) Epidemiology and natural history of VHC infection. Nat Rev Gastroenterol Hepatol 10: 553-562. Link: https://goo.gl/3ELaI4
- Peker KM, Islam (2014) Risk factors associated with high prevalence rates of hepatitis C infection in Egypt. Int J Infect Dis 25: 104-106. Link: https://goo.gl/09AMdq
- World Health Organization (2015) Global Tuberculosis Report 2015. Genva. Link: https://goo.gl/JJ8I9Y
- WHO Media Center/ HIV/AIDS (2014) June 3, 2015. Link: https://goo.gl/xpjBoK
- World Health Organization (2013) Global tuberculosis report 2013.Geneva (Switzerland). Link: https://goo.gl/jA6QNt
- Lorent N, Sebatunzi O, Mukeshimana G, Ende J, Clerinx J (2011) Incidence and risk factors of serious adverse events during antituberculous treatment in rwanda: a prospective cohort study. Plos One 6: e19566. Link: https://goo.gl/O5VgRf
- Richards DC, Mikiashvili T, Parris JJ, Kourbatova EV, Wilson JC, et al. (2006) High prevalence of hepatitis C virus but not HIV co-infection among patientswith tuberculosis in Georgia. Int J Tuberc Lung Dis 10: 396-412. Link: https://goo.gl/yZEbyv
- Ungo JR, Jones D, Ashkin D, Hollender ES, Bernstein D, et al. (1998) Antituberculosis drug-induced hepatotoxicity. The role of hepatitis C virus and the HIV. Am J Respir Crit Care Med 157: 1871-1876. Link: https://goo.gl/qyoCWx
- Chien JY, Huang RM, Wang JY, Ruan SY, Chien YJ, et al. (2010) Hepatitis C virus infection increases hepatitis risk during anti-tuberculosis treatment. Int J Tuberc Lung Dis 14: 616-621. Link: https://goo.gl/juMXxs
- Yew WW, Leung CC (2006) Anti-tuberculosis drugs and hepatotoxicity. Respirology 11: 699-707. Link: https://goo.gl/1S41Gz
- Dye C (2007) Global epidemiology of tuberculosis. Lancet 367: 938–940.
- Schechter M, Zajdenverg R, Falco G, Barnes GL, Faulhaber JC, et al. (2006) Weeklyrifapentine/isoniazid or daily rifampin/pyrazinamide for latenttuberculosis in household contacts. Am J Respir Crit Care Med 173: 922-926. Link: https://goo.gl/iGPcee
- Kunimoto D, Warman A, Beckon A, Doering D, Melenka L (2003) Severe hepatotoxicity associated with rifampin-pyrazinamide preventative therapy requiring transplantation in an individualat low risk for hepatotoxicity. Clin Infect Dis 36: 158-161. Link: https://goo.gl/CK0TfG
- van Hest R, Baars H, Kik S, van Gerven P, Trompenaars MC, et al. (2004) Hepatotoxicity of rifampin-pyrazinamide and isoniazid preventive therapy and tuberculosis treatment. Clin Infect Dis 39: 488-496. Link: https://goo.gl/06pUwc
- Forget EJ, and Menzies D (2006) Adverse reactions to first-line antituberculosis drugs. Expert Opin Drug Saf 5: 231-249. Link: https://goo.gl/kRTpl9
- Younossian AB, Rochat T, Ketterer JP, Wacker J, Janssens JP (2005) High hepatotoxicity of pyrazinamide and ethambutolfor treatment of latent tuberculosis. Eur Respir J 26: 462-464. Link: https://goo.gl/i7Oi20
- Sadaphal JP, Astemborski NM, Graham, Sheely L, Bonds M, et al. (2001) Isoniazid preventive therapy, hepatitis C virus infection, and hepatotoxicity among injection drug users infected with Mycobacterium tuberculosis. Clin Infect Dis 33: 1687-1691. Link: https://goo.gl/cyF2Wp
- Pan L, Jia ZS, Chen L, Fu EQ, Li GY (2005) Effect of anti-tuberculosis therapy on liver function of pulmonary tuberculosis patients infected with hepatitis B virus. World J Gastroenterol 11: 2518-2521. Link: https://goo.gl/lAHpOu
- Agal S, Baijal R, Pramanik S, Patel N, Gupte P, et al. (2005) Monitoring and management of antituberculosis drug induced hepatotoxicity. J Gastroenterol Hepatol 20: 1745-1752. Link: https://goo.gl/XgOnHl
- Fouelifack F, Keugoung B, Fouedjio J, Kouam N, Mendibi S, et al. (2012) High Rates of Hepatitis B and C and HIV Infections among Blood Donors in Cameroon, A Proposed Blood Screening Algorithm for Blood Donors in Resource Limited Settings. Journal of Blood Transfusion Article ID 458372. Link: https://goo.gl/VA2uWX
- WHO, (2013) WHO urges governments to act on hepatitis threat [Internet]. Link: https://goo.gl/oZbu6r
- Nail AM, Ahmed NE, Gaddour MOE (2013) Seroprevalence of hepatitis B and C viruses among tuberculosis patients. Sudan Journal of Medical science 8: 17-22. Link: https://goo.gl/Kms3RV
- Sirinak C, Kittikraisak W, Pinjeesekikul D, Charusuntonsri P, Luanloed P, et al. (2008) Viral hepatitis and HIV–associated tuberculosis: Risk factors and TB treatment outcomes in Thailand. BMC Public Health 8: 245. Link: https://goo.gl/uRk8OI
- Kuniholm MH, Mark J, Aladashvili M, Shubladze N, Khechinashvili G, et al. (2008) Risk factors and algorithms to identify hepatitis C, hepatitis B,and HIV among Georgian tuberculosis patients. Int J Infect Dis 12: 51-56. Link: https://goo.gl/fjt6m8
- Akhtar I, Qamar MU, Hakeem A, Waheed A, Sarwar F, et al. (2013) Seroprevalence of VHB and VHC at tuberculous patients at Sheikh Zayed Hospital Rahim Yar Khan, Pakistan. Biomedica 29: 69-72.
- Abdallah TM, Idriss MI, Ahmed AM, Ali AA, Saeed OK (2015) Sero-Prevalence of Hepatitis B and Hepatitis C Viruses among Tuberculosis Patients in Kassala, Eastern Sudan. Glob J Infect Dis Clin Res 1: 001-003. Link: https://goo.gl/sRkEqT
- Ymele F, Keugoung B, Fouedjio JH, Kouam N, Mendibi S, et al. (2012) High rates of Hepatitis B and C and HIV infections among blood donors in Cameroon: A proposed blood screening algorithm for blood donors in resource-limited settings. J Blood Transfus. Link: https://goo.gl/ZOjBwR
- Blal CA, Passos SR, Horn C, Georg I, Bonecini-Almeida MG, et al. (2005) High prevalence of hepatitis B virus infection among tuberculosis patients with and without HIV in Rio de Janeiro, Brazil. Eur J Clin Microbiol Infect Dis 24: 41-43. Link: https://goo.gl/fRDNqS
- Aires RS, Matos MA, Lopes CL, Teles SA, Kozlowski AG, et al. (2012) Prevalence of hepatitis B virus infection among tuberculosis patients with or without HIV in Goiânia City, Brazil. J Clin Virol 54: 327-331. Link: https://goo.gl/R49NzV
- Abdlah TM, Mohammed MH, ALI AA (2011) Seroprevalence and epidemiological factors of hepatitis B virus (VHB) infection in Eastern Sudan. International Journal of Medicine and Medical Sciences 3: 239-241. Link: https://goo.gl/OP7CGR
- Nooredinvand HA, Connell DW, Asgheddi M, Abdullah M, O'Donoghue M, et al. (2015) Viral hepatitis prevalence in patients with active and latent tuberculosis. World J Gastroenterol 21: 8920-8926. Link: https://goo.gl/rgx7GN
- Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H (2014) Global epidemiology and genotype distribution of the hepatitisC virus infection. J Hepatol 61: S45-S57. Link: https://goo.gl/o2y8oS
- Reis NR, Lopes CL, Teles SA, Matos MA, Carneiro MA, et al. (2011) Hepatitis C virus infection in patients with tuberculosis in Central Brazil. Int J Tuberc Lung Dis 15: 1397-1402. Link: https://goo.gl/K1MyTX
- Richards D, Mikiashvili T, Parris JJ, Kourbatova EV, Wilson JCE, et al. (2006) High prevalence of hepatitis C virus but not HIV co-infection among patients with tuberculosis in Georgia. Int J Tuberc Lung Dis 10: 396-401. Link: https://goo.gl/zT5MDq
- Agha MA, EL-Mahalawy II, Seleem HM, Helwa MA (2015) Prevalence of hepatitis C virus in patients with tuberculosis and its impact in the incidence of anti-tuberculosis drugs induced hepatotoxicity. Egyptian Journal of Chest Diseases and Tuberculosis 64: 91-96. Link: https://goo.gl/JrTQz5
- Khalili H, Khavidaki S, Mehrnaz R, Rezaie L, Etminani M (2009) Anti-tuberculosis drugs related hepatotoxicity; incidence, risk factors, pattern of changes in liver enzymes and outcome. J Pharm Sci 17: 163-167. Link: https://goo.gl/IYYp06
- Abdallah TM, Ali AA (2012) Sero-prevalence of transfusion-transmissible infectious diseases among blood donors in Kassala, eastern Sudan. Journal of Medicine and Medical Science 3: 260-262. Link: https://goo.gl/2TtmKH
- Khalid H, Idris, Bahaeldin K, Elamin (2015) Seroprevalence of hepatitis b (hbs ag) and hepatitis c (anti-VHC) viruses among sudanese patients with hiv/tb co-infection, International Journal of Information Research and Review. 2: 765-768. Link: https://goo.gl/jhGilQ
- Endale M, Tekle A, Edessa N, Mulugeta K (2015) Prevalence of triple viral infections of human immunodeficiency virus (HIV), hepatitis B and C among tuberculosis patients and associated risk factors: The case of West Arsi Zone, Ethiopia. African Journal of Microbiology Research 9: 1675-1683. Link: https://goo.gl/BlEyi2
- Tahziba H, KulshreshthaKK, YadavVS, Kiran K (2016) HIV and VHB co-infections among patients with active TB disease attending a primary health carecentre in a rural area of north India. Egyptian Journal of Chest Diseases and Tuberculosis 65: 227-232. Link: https://goo.gl/Ag3Lm3
- SubIr KD, InDranath G, DebojyotI B, Praveen A, SuManta J, et al. (2013) Liver Function Profile Anomalies in HIV Seropositive Tuberculosis. J Clin Diagn Res 7: 1068-1072. Link: https://goo.gl/7Pz0MY
- Padmapriyadarsini C, Chandrabose J, Victor L, Hanna LE, Arunkumar N, et al. (2006) Hepatitis B or hepatitis C co-infection in individuals infected with human immunodeficiency virus and effect of anti-tuberculosis drugs on liver function. J Postgrad Med 52: 92-96. Link: https://goo.gl/UV7jMe
- Oluwafemi O, Oguntibeju B, Olatubosan B (2003) A Study on the Activities of Liver Enzymes in HIV/AIDS Patients. J Med Sci 3: 106-109.
- Wild-Up M, Fortuin F, Whittle DHC, Hall AJ, Wolf CR, Montesonai R (1990) Liver and hepatitis Bvirusin Gambian children. Cancer Epidemiol Biomarkers. Prev 2: 555-561.
- Wit FW, Weverling GJ, Weel J, Jurrian S, Lange JM (2002) Incidence of and risk factors for severe hepatotoxity associated with antiretroviral combination therapy. J Infect Dis 186: 23-31. Link: https://goo.gl/Tfb55J
- Margulis SJ, Honig CL, Soave R, Govoni AF, Mouradian JA, et al. (1986) Biliary tract Obstruction in the AIDS. Ann Intern Med 105: 207-210. Link: https://goo.gl/ySMEfE
- Lomtadze N, Kupreishvili L, Salakaia A, Vashakidze S, Sharvadze L, et al. (2013) Hepatitis C Virus Co-Infection Increases the Risk of Anti-Tuberculosis Drug-Induced Hepatotoxicity among Patients with Pulmonary Tuberculosis. PLoS ONE 8: e83892. Link: https://goo.gl/Ta41oW
- Lee BH, Koh WJ, Choi MS, Suh GY, Chung MP, et al. (2005) Inactive Hepatitis B Surface Antigen Carrier State and Hepatotoxicity DuringAntituberculosis Chemotherapy. Chest 127: 1304-1311. Link: https://goo.gl/NjAEzU
- Ayelagbe OG, Akerele OP, Onuegbu AJ, Oparinde DP (2014) Drug Hepatotoxicity in HIV Patients on Highly Active Antiretroviral Therapy [HAART] in Southwest Nigeria. IOSR Journal of Dental and Medical Sciences 13: 67-70. Link: https://goo.gl/tsjwWa
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
