Global Journal of Infectious Diseases and Clinical Research
Survey on the Prevalence of Buruli Ulcer in Patients with Chronic Wounds in Pointe-Noire
Freddy Saturnin Pouki1-3*, Aubierge Victoire Kimpamboudi Matondo4, Parode Ragive Takale1,3, Constant Biocat5, Rodiano Tcibinda3, Siméon Nama3, Steven Moukalath6, Axelle Paquet7, Jery Steve Ferole Boungou7 and Luc Magloire Anicet Boumba1,8,9
1Faculty of Health Sciences, Marien Ngouabi University, BP: 69 Brazzaville, Congo
2Tié Tié Referral Hospital, Pointe-Noire, Congo
3Guénin Medical Clinic, Pointe-Noire, Congo
4Departmental Directorate of Care and Health Services, Pointe-Noire, Congo
5Général Adolph Sicé Hospital, Pointe-Noire, Congo
6Operational Sector, Pointe-Noire, Congo
7Perenco-Congo Local Relations and Impact Department, Pointe-Noire, Congo
8Pointe-Noire Research Zone, National Institute for Research in Health Sciences (IRSSA) Brazzaville, Congo
9Medical and morphological analysis laboratory, Loandjili General Hospital, Pointe-Noire, Congo
Cite this as
Pouki FS, Kimpamboudi Matondo AV, Takale PR, Biocat C, Tcibinda R, Nama S, et al. Survey on the Prevalence of Buruli Ulcer in Patients with Chronic Wounds in Pointe-Noire. Glob J Infect Dis Clin Res. 2024;10(1): 017-022. Available from: 10.17352/2455-5363.000062Copyright
© 2024 Pouki FS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Background & objective: Buruli Ulcer (BU) is a human skin infection caused by Mycobacterium ulcerans, an environmental mycobacterium. This pathology is most prevalent in humid tropical regions, particularly near swampy or flood-prone areas. In Pointe-Noire, a coastal city in the Republic of Congo, Buruli ulcer is a health threat requiring ongoing surveillance and awareness-raising efforts to control its spread. The aim of this study was to determine the prevalence of Buruli ulcers in patients consulting for chronic wounds in Pointe-Noire.
Methods: This was a descriptive cross-sectional study conducted from June 10, 2023 to September 18, 2024. We recruited 69 patients with chronic wounds consulted at the Adolphe Sicé General Hospital, the Tié Tié Referral Hospital, and the Pointe Noire Operational Sector. Mycobacterium ulcerans was systematically detected using the qPCR technique targeting the M. ulcerans IS 2404 specific insertion element on swab samples.
Results: 69 patients with suspected BU were enrolled, with 57% (39) male and 43% (30) female subjects. The mean age was 47.7 ± 18.5, ranging from 6 to 94 years, and the most represented age group was [30-60] (66.7%). The wounds observed in our patients were mainly ulcerative in 94.2% (65) and localized to the lower limbs in 97.1% (67). The overall prevalence of Mycobacterium ulcerans infection was 2.89%. The age group affected was < 15 years, with 01 positive male case and [30-60], with 1 positive female case.
Conclusion: The study carried out in Pointe-Noire revealed a Buruli ulcer prevalence of 2.86% among the 69 patients with chronic wounds tested, with 02 positive cases.
Introduction
Buruli ulcer (BU) Mycobacterium ulcerans is a cosmopolitan environmental mycobacteriosis affecting humans, particularly in intertropical environments. This emerging disease, cases of which were described in the Buruli region of Uganda in 1962 [1]. Since 2003, Buruli ulcer has been classified as a neglected tropical disease (NTD) [2]. It has been reported in 33 countries in Africa, the Americas, Asia and the Western Pacific. With the exception of Australia and Japan, most cases occur in tropical and subtropical regions. Until 2010, the number of suspected cases of Buruli ulcer notified each year worldwide was around 5,000, but then declined until 2016, when 1961 cases were reported, the lowest level ever recorded. Thereafter, the number of cases rose again each year, reaching 2,713 cases in 2018. Since then, the number of cases has fallen in 2019 (2271), 2020 (1458) and 2021 (1370). The decreases observed in 2020 and 2021 could be attributable to the effects of COVID-19 on active case detection activities [3]; or may reflect the positive effects of control programmes or the collateral effects of other health programmes. Buruli ulcer is most often initially manifested as a mobile, painless nodule. It can also appear as an extensive area of induration or diffuse swelling on the arms or legs. The disease progresses without fever or pain, which partly explains why sufferers are often slow to seek treatment.
Eventually, a massive, classic ulcer with sunken edges appears. ‘Sometimes the bone is affected, causing enormous deformities. When the lesions heal, scarring results in restricted limb movement or other permanent disabilities in a quarter of patients. This tissue destruction is caused by a toxin produced by Mycobacterium ulcerans. In the absence of early treatment with appropriate antibiotics, the disease causes lasting functional disabilities as well as significant cosmetic handicaps [4].
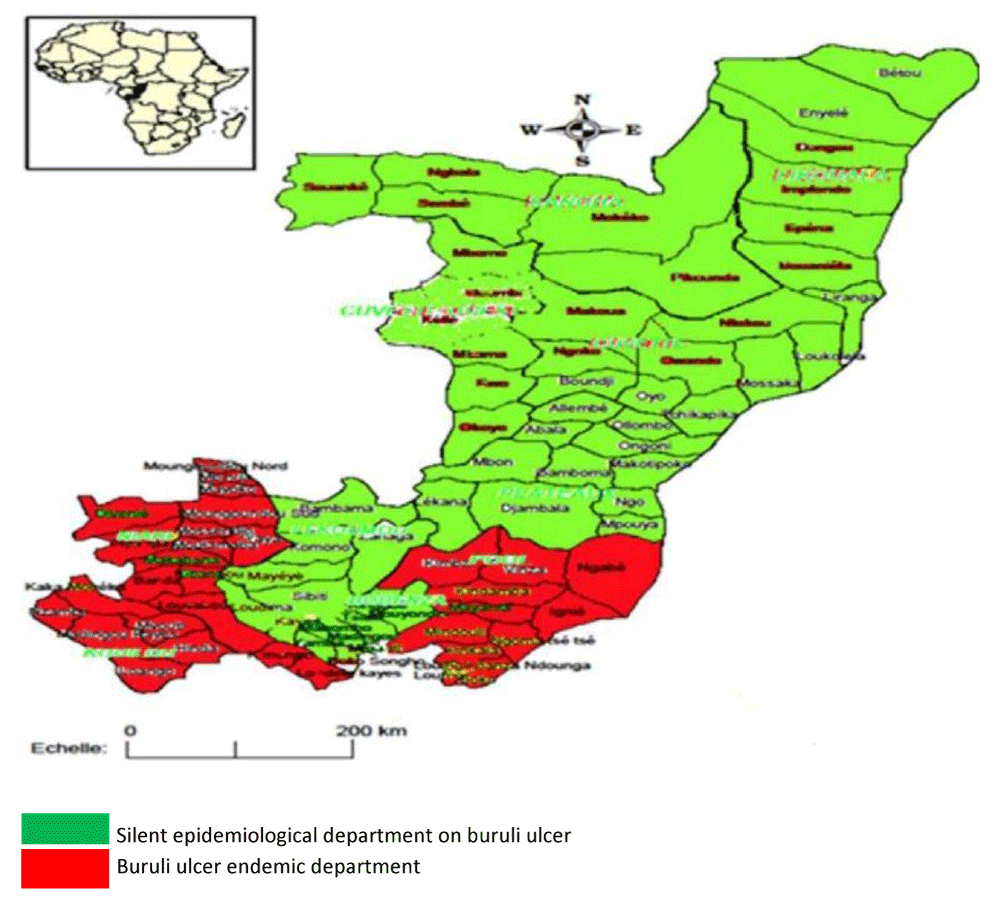
In the Congo, the fight against Buruli ulcer dates back to 2006, when the first cases were discovered (370 cases), particularly in the departments of Pointe-Noire (9 cases), Kouilou (296 cases), Niari (36 cases) and Bouenza (29 cases). Incidence is expected to peak in 2021 with 189 cases, with a relative drop to 40 cases in 2022. Although the pockets of endemicity are well known, there are classic foci such as the departments of Kouilou and Niari (Figure 1) and new foci in the departments of Likouala and Sangha [5,6].
In all low-resource countries, chronic wounds are a major public health challenge because they are often a source of significant functional disability, pain, and social stigma. They are also synonymous with high healthcare costs and loss of employment and income. Pointe-Noire, a coastal town, is endemic for Buruli ulcer (Figure 1), a source of particularly debilitating chronic wounds. It is in this context that we propose to determine the prevalence of Buruli ulcers in patients consulting for chronic wounds in Pointe-Noire.
Materials and methods
Type and period of study
This was a descriptive cross-sectional study with prospective data collection, carried out from 10 June 2023 to 18 September 2024 under the leadership of the Pointe-Noire Departmental Directorate of Health Care and Services, with the support of Perenco-Congo’s Local Relations and Impact Department.
Population and study sites
A total of 69 patients consulted or hospitalised for chronic wounds at the Adolphe Sicé General Hospital, the Tié Tié Referral Hospital, and the Pointe Noire Operational Sector.
Inclusion and non-inclusion criteria
The epidemiological and clinical Buruli ulcer score was according to WHO standards. It involved scoring from 1 to 3 on the following ten elements: the patient’s age and usual place of residence, the characteristics, location, number, course, and age of the lesion, and the presence or absence of spontaneous pain, fever, and adenopathy. The sum of the scores then gives a final score with the following conclusions: 10-13: diagnosis unlikely1, 14-16: unlikely, 17-20: likely, 21-24: very likely.
All consenting patients with a score ≥ 17 were included. Non-inclusion was validated for all non-consenting patients and all patients with a score < 17.
Clinical survey
Data such as age, sex, type, and location of lesions were collected using a survey form.
Biological investigation
Laboratory analyses were carried out in the Molecular Biology Laboratory of the Guenin Medical Clinic in Pointe Noire.
Sampling: Using cotton wool impregnated with alcohol or disinfectant, the skin surrounding the ulcer was carefully cleaned twice, avoiding the base of the ulcer; at least two swabs were taken depending on the lesions, and gently inserted under the edges of the ulcer; the swab was rolled back and forth and the tissue was rubbed under the edge of the ulcer in a clockwise motion; the swab was then carefully inserted into the citoswab Transport Medium (W/3ML VTM) supplied by CITOTEST LABWARE MANUFACTURING CO. LTD Haimen city 226100, China; the VTM was labelled with the name, age, and sex of the patient and the date the sample was obtained.
Molecular analysis
a) Extractions: After microbiological deactivation of the samples at +80°c in a water bath for 20 min, we first extracted the DNA using the Nzytech gDNA Isolation kit according to the following protocol:
- Pre-lysis: The sample of 200 µL was placed in an Eppendorf tube and 5 µL of Internal Control and 180µL of Buffer NT1 with 25 µL of proteinase K was added to each sample. Incubation was at 56 °C for 1 to 3 hours, vortexing occasionally.
- Lysis: After the vortex mix, 200µL of Buffer NL was added then it was vortex mixed for 10 seconds and allowed to stand for 10 Minutes, and 210µL of Ethanol (100%) was added then vortex immediately.
- DNA fixation: The lysate was transferred from each sample to the filters (Nzyspin tissue column) and placed in a 2 mL collection tube. Centrifuged for 1 minute at 11,000g then the flow-through was discarded and the filter was placed in a new collection tube.
- Purification with silica membrane: Added to the column was 500 μL of NW1 buffer, centrifuged for 1 minute at 11,000g; the flow-through was discarded and the filter was placed in a new collection tube. Next, 600 μL of buffer NW2 (make sure the ethanol has been added beforehand) was added, and centrifuged for 1 min at 11,000 g, the flow-through was discarded afterward, and the filters (NZYSpin Tissue Column) returned to the collection tube and centrifuge for 2 min at 11,000g to dry the Silica Membrane thoroughly.
- DNA elution: The column was first placed in a clean microcentrifuge tube (Eppendorf tubes) and then 100 μL of NE buffer (pre-warmed to 70°C to improve the yield), incubated for 1 min at room temperature and centrifuged at 11,000 g for 2 min to elute the DNA. Genomic DNA can be stored at 4 °C or -20 °C.
b) Amplification: The extracted DNA underwent RT-PCR using the Genesig Advanced Kit, targeting the M. ulcerans IS 2404 insertion sequence.
- The first step consisted of preparing the Mix according to the following protocol:
-10 µl PrecisionPlus 2XqPCR Master Mix
-01 µl M. ulcerans primer/probe
-04 µl nuclease-free water
-05 µl DNA - The second step was to program the Mic (thermocycler) according to the data in Table 1. And the choice of targets according to the guidelines in Table 2. The Pcr lasted only 1h40min 46sec.
- The third step consisted of interpreting the results according to the information in Table 3.
Statistical analysis
The categorical data are expressed as numbers (percentages) and variables. All analyses were conducted using SPSS software (version 26.0; IBM).
Results
Sociodemographic data
The study population consisted of 69 subjects, 57% (39) of whom were male and 43% (30) female. The sex ratio (M/F) was 1.18 (Table 4). The mean age was 47.7 ± 18.5, ranging from 6 to 75 years, and the age group most represented was [30-60], with a proportion of 66.7%.
Clinical characteristics
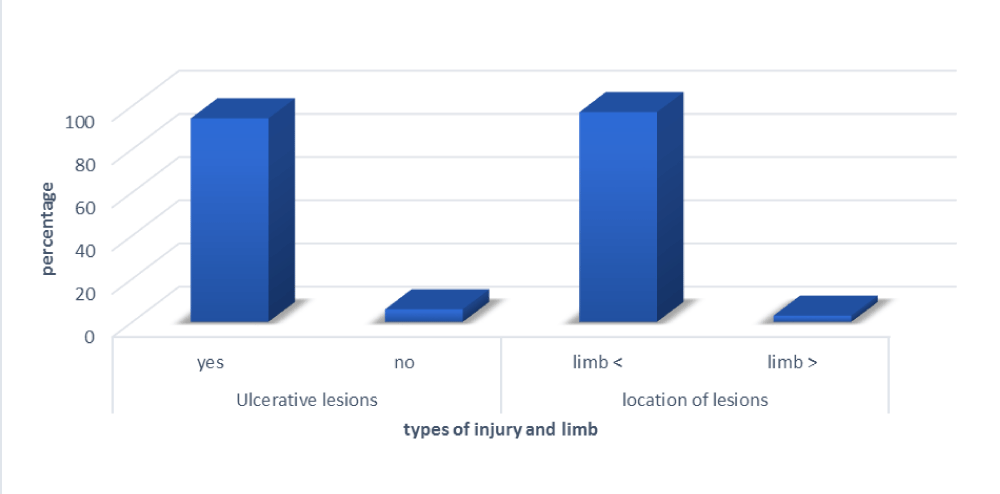
The wounds observed in our patients were mainly ulcerative in 94.2% (65) and located on the limbs in 97.1% (67) (Figure 2).
Prevalence of Mycobacterium ulcerans (Burili ulcer)
The overall prevalence of Mycobacterium ulcerans infection is 2.89% (Table 5). The age group affected was <15 years, with 01 positive male case, and [30-60], with 1 positive female case (Figure 3).
Discussion
Climate change leads to changes in the environment, which in turn affects the geographical distribution of living organisms. Bairnsdale or Buruli ulcer is an infectious necrotising skin disease caused by Mycobacterium ulcerans, an environmental mycobacterium. Early case detection, accurate diagnosis, and treatment with antibiotics are currently the first-line recommendations for the management of Buruli ulcer [7]. Buruli ulcer surveillance is a public health objective both to measure the importance and the evolution of the incidence in Pointe Noire. This surveillance is important to better document the burden of the disease, to map endemic areas, and also to ensure better case management. We, therefore, conducted this study with the aim of determining the prevalence rate of Buruli ulcer in patients with chronic wounds in Pointe-Noire.
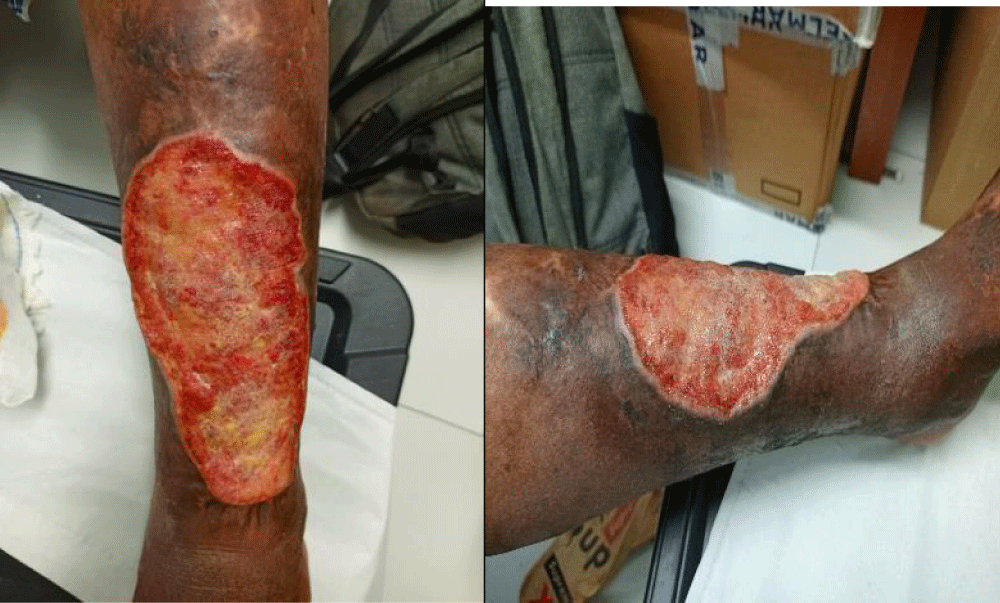
The majority of patients included in our study had been detected at ulceration stage 65 (94.2%). These were old lesions that were progressing towards complications (Figure 4). Similar results have been described by Iabichella. Ml, et al. 2015; and Anagonou. Eg, et al. 2019 in Benin [8,9]. This may be explained by delays in diagnosis, prioritisation of traditional treatments, late consultations, and diagnostic errors by healthcare staff with little training in detection at the pre-ulcer stage.
As regards the location of the lesions, they predominated in the lower limbs, found in 67 (97.1%) of patients. This is a classic expression, explained by the more frequent contact of water with the lower limbs when immersed in contaminated water. Similar profiles have been described by GA.Aloumba, et al. 2024 in Congo [10].
Our survey found a prevalence of Buruli ulcer of 2.89% among the 69 patients tested, with 02 positive cases. Our result is similar to that found by Marion E.; 2015 in Bounou, Benin [11]. This can be justified by the finding of Zingue D, et al. 2018 [12] that our country is currently one of the Buruli ulcer hotspots in Central Africa.
In our work, we found that the age group affected was <15 years, with 01 positive male case, and [30-60], with 1 positive female case. This series showed no link between Buruli ulcer, sex, and age. These data do not corroborate those carried out by Boyd SC, et al. 2012 [13] in an Australian population and in Kouilou, Congo by Marion E, et al. 2014 [5] whose 108 positives were represented by 60 (56%) females and 48 (44%) males; 56% of case-patients were aged < 15 years. In general, there is no difference between boys and girls in the rate of UB infection [4] and it can affect all age groups [14].
Buruli ulcer is the third most common mycobacterial disease, after tuberculosis and leprosy, and is caused by Mycobacterium ulcerans, which produces a mycolactone toxin responsible for tissue destruction, leading to extensive skin ulcerations. However, chronic wounds resulting from Buruli ulcer are often difficult to treat due to the persistence of the infection and resistance to antibiotics. This can lead to serious complications, including secondary infections and prolonged healing [4].
The detection of positive cases, even in small numbers, indicates the presence and circulation of Mycobacterium ulcerans in the local environment. The low prevalence observed should not detract from the importance of prevention and treatment efforts, given the potentially serious consequences of the disease. However, it has still been necessary to take samples other than swabs to increase the chance of identifying the germ. Such as biopsies, because, according to Ibrahim Yl, et al. 2019 [15], punch biopsies make it possible to establish the correct diagnosis of Buruli ulcer and also the differential diagnosis of chronic ulcers. This reduction may also reflect the limitations of the clinical diagnosis at the time of inclusion and the insignificant size of the study subjects.
Conclusion
The study conducted in Pointe-Noire revealed a prevalence of Buruli ulcer, with 2 positive cases out of 69 individuals tested, i.e. approximately 2.86%. The positive individuals belonged to various age groups, with no significant gender predominance. Although the number of cases is limited, this finding highlights the importance of ongoing vigilance and epidemiological surveillance to prevent a possible increase in infections.
- Weir E. Buruli ulcer: the third most common mycobacterial infection. CMAJ. 2002;166(13):1691. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC116159/
- Kempf M, Johnson RC, Marsollier L, Marion E. Buruli ulcer, a neglected tropical disease due to Mycobacterium ulcerans. French-speaking Review of Laboratories. 2023;2023(556):43-50. Available from: https://doi.org/10.1016/S1773-035X(23)00214-9
- World Health Organization. Buruli ulcer (Mycobacterium ulcerans infection). Accessed October 12, 2024. Available from: https://www.who.int/news-room/fact-sheets/detail/buruli-ulcer-(mycobacterium-ulcerans-infection)
- Nau JY. Buruli ulcer: vector identified. Rev Med Suisse. 2008. Available from: https://www.revmed.ch/revue-medicale-suisse/2008/revue-medicale-suisse-152/ulcere-de-buruli-vecteur-identifie
- Marion E, Obvala D, Babonneau J, Kempf M, Asiedu KB, Marsollier L. Buruli ulcer disease in Republic of the Congo. Emerg Infect Dis. 2014;20(6):1070-1072. Available from: https://doi.org/10.3201/eid2006.131498
- World Health Organization. Master Plan for the fight against NTDs Congo 2023-2027. Accessed October 14, 2024.
- Quaye C, Abel AA, Gyamfi E, Dogbe M, Sarpong DM, Twumasi-Mensah T, et al. Integrating mobile phone and social media interventions in health research to improve Buruli ulcer detection in Ghana and Côte d’Ivoire. WHO. 2019. Available from: https://iris.who.int/bitstream/handle/10665/329375/WHO-CDS-NTD-IDM-2019.01-fre.pdf
- Iabichella ML, Salmon O, Bertolotti A, Izzo A, Fusari V, Lugli M. Buruli ulcer: management in hospital or at public health centers in "brousse". Angéiologie. 2015;67(1):29-41. Available from: https://www.medicalmate.gr/wp-content/uploads/2020/06/6-029-041-Iabichella-BuruliFR.pdf
- Anagonou EG, Biaou CA, Wadagni AC, Barogui YT, Ayelo GA, Houezo J, et al. Evolution of Buruli ulcer in the Atlantic and Littoral departments (South Benin) from 2008 to 2018. Med San Pub. 2019;15:1-18. Available from: https://www.researchgate.net/profile/Ghislain-Sopoh/publication/336799326_1ANAGONOU/links/5db2c38992851c577ec27076/1ANAGONOU.pdf
- Aloumba GA, Ondima IPL, Bayonne KES, Niama RF-Tchatchouang S, Ndziessi G, et al. Bull. de l’ALLF. 2024;38: November. Available from: https://ballf.fr/wp-content/uploads/2024/10/BALLF-38-Ulcere-de-Buruli.pdf
- Marion E, Carolan K, Adeye A, Kempf M, Chauty A, Marsollier L. Buruli ulcer in South Western Nigeria: a retrospective cohort study of patients treated in Benin. PLoS Negl Trop Dis. 2015;9(1):e3443. Available from: https://doi.org/10.1371/journal.pntd.0003443
- Zingue D, Bouam UN, Tian RBD, Drancourt M. Buruli Ulcer, a Prototype for Ecosystem-Related Infection, Caused by Mycobacterium ulcerans. Clin Microbiol Rev. 2018;31(10):e00045-17. Available from: https://doi.org/10.1128/cmr.00045-17
- Boyd SC, Athan E, Friedman ND, Hughes A, Walton A, Callan P, et al. Epidemiology, clinical features and diagnosis of Mycobacterium ulcerans in an Australian population. Med J Aust. 2012;196(5):341-344. Available from: https://doi.org/10.5694/mja12.10087
- Yotsu RR, Murase C, Sugawara M, Suzuki K, Nakanaga K, Ishii N, et al. Revisiter l'ulcère de Buruli. J Dermatol. 2015;42:1033-1041. Available from: https://doi.org/10.1111/1346-8138.13049
- Ibrahim YL, Masouye I, Tschanz E, Atangana P, Etard JF, Serafini M, et al. Diagnostic Value of Histological Analysis of Punch Biopsies in Suspected Cutaneous Buruli Ulcer: A Study on 32 Cases of Confirmed Buruli Ulcer in Cameroon. Dermatopathology. 2019;6:28-36. Available from: https://doi.org/10.1159/000498969
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.



 Save to Mendeley
Save to Mendeley
