Global Journal of Infectious Diseases and Clinical Research
Parasitological Consequences Followed Dysentery Infections in Mesopotamia
Mays Kadhim Oleiwi and Samadov Aabdic*
Cite this as
Oleiwi MK, Aabdic S. Parasitological Consequences Followed Dysentery Infections in Mesopotamia. Glob J Infect Dis Clin Res. 2024;10(1): 012-016. Available from: 10.17352/2455-5363.000061Copyright
© 2024 Oleiwi MK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.When compared to the opposing addresses in this research report, the present investigation revealed significant data (p < 0.05) about dysenteric infection rates in male patients, elderly patients, and rural places. Additionally, the infections with dysenteric agents were demonstrated using GSE and deoxycholate citrate agar media. The hypothesis is positioned beneath the statistical pyramid probability center by more structural research.
Introduction
Dysentery is a gastrointestinal ailment causing severe diarrhea with blood or mucous, abdominal discomfort, fever, incomplete feces, and potential complications like dehydration [1].
Dysentery mostly comes in two forms:
- Amoebic dysentery, also known as amoebiasis, is primarily caused by the parasite Entamoeba histolytica, while other parasites include Balantidium coli and Strongyloides stercoralis.
- Bacillary dysentery is a common bacterial infection caused by Shigella, Salmonella, Campylobacter, and Escherichia coli, with E. coli being the most common type.
Distemper can be lethal if left untreated. See your healthcare practitioner if you experience any dysentery symptoms or diarrhea, caused by Shigella or Entamoeba bacteria, chemicals, protozoa, or parasitic worms, which can spread between individuals due to fecal contamination from improper sanitation, leading to gastrointestinal inflammation [2].
Traveling to high-risk countries can prevent dysentery by practicing handwashing and food safety precautions, and ensuring adequate water or oral rehydration solution during the illness’s recovery period [3].
Shigella causes 1.1 million deaths and 165 million diarrhea cases annually, primarily in underdeveloped nations. Entamoeba histolytica causes over 50% of diarrhea cases in unsanitary regions, causing millions of misery and 55,000 fatalities [4].
Dysentery, a prevalent condition with over 1.7 billion reported cases globally, varies in symptoms depending on the type of illness.
Amoebic dysentery
The majority of cases of amoebic dysentery are symptomless.
Mild symptoms of amoebic dysentery may include: Diarrheal disease, High body temperature, Stooling and nausea, and a decrease in weight. , Distressed abdomen. In rare circumstances, the parasite may spread to other bodily parts and cause an abscess [3].
Bacillary dysentery
Bacillary dysentery can cause the following symptoms: Bloody or mucusy diarrhea, High body temperature, vomiting and nauseous, Severe cramping in the stomach (abdominal pain), severe renal damage, big intestinal dilatation, and severe inflammation are possible consequences of severe dysentery.
Bacillary dysentery is a common type of dysentery, characterized by moderate symptoms like loose stools, diarrhea, and stomach aches. It’s caused by bacteria affecting bowel movements, feces, blood, pus, or mucus. Caustic situations may include severe abdominal cramping, shock, fever, and disorientation [5].
In hazardous situations, individuals may pass over one liter of liquid in an hour, often experiencing bloody diarrhea, low-grade fever, and abdominal discomfort. Dysentery can also cause rapid weight loss and muscle pains, potentially leading to systemic infection.
An occasional bloodstream infection by the amoebic parasite might cause it to go outside of the intestines. In such circumstances, it may more dangerously infect other organs, including the liver, brain, and lungs [6].
Dysentery is a highly contagious disease caused by bacterial and parasitic diseases. Human-to-human transmission of parasites or germs often occurs when an infected person’s feces enter the mouth of another. Transmission may take place when: Food is prepared by someone with inadequate hygiene or without washing their hands, Consuming tainted water, or intercourse, particularly when it concerns the anus.
When foreign germs infiltrate your body and cause a serious infection, you have bacillary dysentery. Bacteria that frequently cause bacillary dysentery include the following:
Shigella, which leads to shigellosis. , Salmonella, which causes salmonella, Campylobacter, which causes campylobacteriosis and Escherichia coli which leads to E. coli infection.
A certain type of parasite that enters your body might cause amoebiasis. Amoebiasis is caused by a parasite, and a stool culture is ordered by a healthcare practitioner. A specific container and one-use spoon are provided. To collect waste, line toilet rims or defecate into an alternative container [7].
Your doctor may recommend a sigmoidoscopy to confirm or rule out alternative symptoms, using a specialized scope to examine the colon and lower rectum [5].
A quick examination and a history can be used to make an analysis. Hematochezia, or the passing of new blood via the anus, usually in or through feces, is not to be mistaken for dysentery.
The skin, mouth, and lips may appear dry due to dehydration. Lower abdominal tenderness may also be present [7].
Oral rehydration is used to maintain fluids in dysentery patients but may require hospital admission if vomiting or diarrhea prevents treatment. Antimicrobial medication should be initiated after identifying the illness, and in limited laboratory access, a combination of antibiotics and amoebicidal treatment may be necessary [8].
Shigellosis can be treated with antibiotics like TMP-SMX or ciprofloxacin, but public antibiotics are losing effectiveness in many forms, and impoverished nations, effective drugs may be difficult to find [9].
Two antimicrobial medications, such as metronidazole and paromomycin or iodoquinol, are frequently used to treat amoebic dysentery. In 2013, Shigella is thought to have killed 40,000 adults over the age of five and 34,000 children under the age of five. Over 50 million individuals contract amoebiasis annually and 50,000 of them pass away (one per thousand) [10].
Materials and methods
1. Location of procedure
From March to October 2023, this process was carried out in the Health and Medical Technical College laboratories of Al-Forat Al-Awsat Technical University in Kufa.
2. Case model
In our study, 381 patients of various ages and genders were included. After receiving a diagnosis in the hospital, patients had their samples collected, put in the appropriate tubes and containers, and then taken to the lab.
3. Sample collection
381 cases’ worth of feces and blood were taken, analyzed in the lab, and then separated into several categories to provide information on the data findings.
4. Cultural medium
The selective medium known as Deoxycholate Citrate Agar (DCA) is used to grow Shigella dysenteriae and other Shigella species, as well as Salmonella spp. It is obtained from commercial scientific offices through digital marketing and contains a concentration of deoxycholate and citrate salts that inhibit the growth of most intestinal flora and many gram-positive bacteria. It was produced in the USA by Biocompare, Inc.
5. Stool examination
To determine the organism causing dysentery, cultures of stool samples are analyzed. Because the quantity of amoebae varies every day, it is usually necessary to collect many samples.
6. Hematological tests
Abnormalities in the levels of blood cells were measured using blood samples.
7. Clinical chemistry
Deviations in the concentrations of vital minerals and salts can be detected by blood testing.
8. Histopathological tests
Stool section slides were inspected under a compound microscope to look for tissues and cells damaged during amebiasis.
9. Statistics
The T-student test was used to validate the confidence probability, and following [11], the interpretation requested an SPSS test.
10. Demographic map
A mapping tool that displays information on the amoeba-infected population in Iraq in 2018.
Results
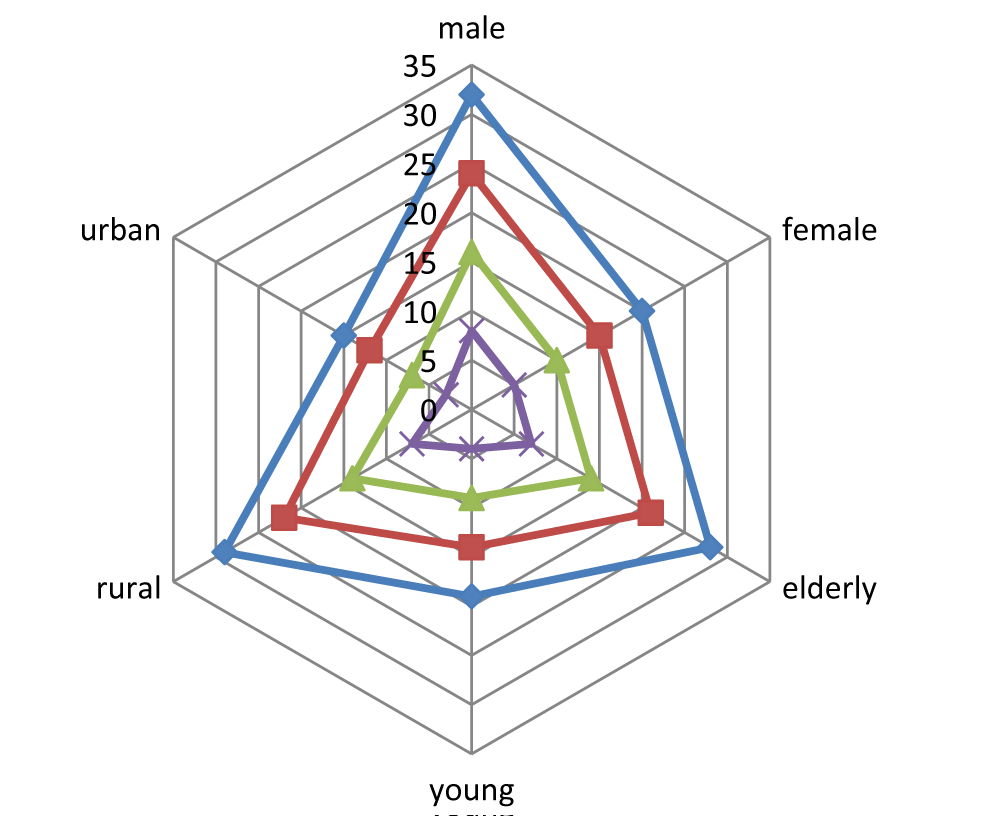
The results of the biostatic analysis showed why the likelihood range for male dysenteric infection cases was less than that of female cases.
When comparing the probability of dysenteric infections, older individuals are more impacted than younger ones (p < 0.05).
In a similar vein, the population of rural areas reports a higher rate of dysenteric injuries than that of urban people (p < 0.05).
“Figure 1” shows the statistical examination of the distribution of dysentery based on many factors.
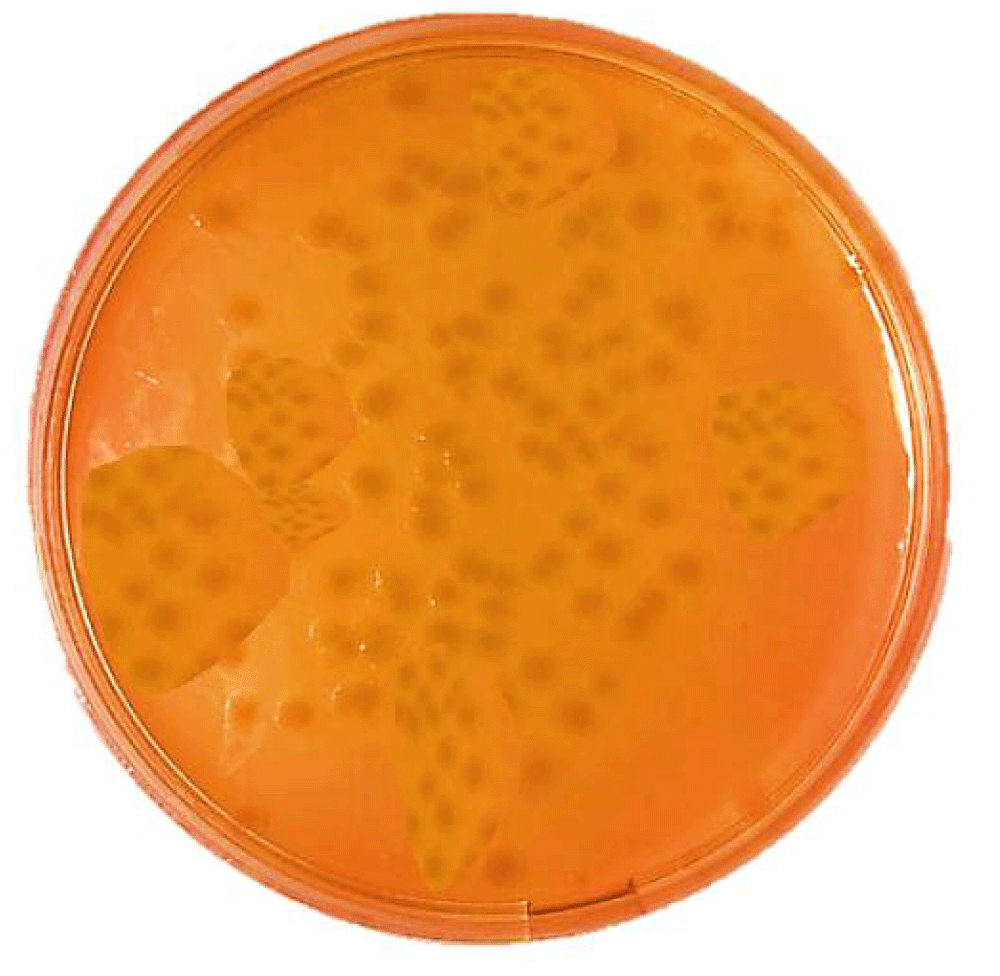
Sghigella dysenteriae colonies in the Deoxycholate Citrate Agar medium are colorless as a result of non-lactose fermentation (NLF). The colonies of Shigella dysenteriae in Salmonella Shigella Agar medium are colorless (Figure 2). Shigella dysenteriae does not create hydrogen sulfide.
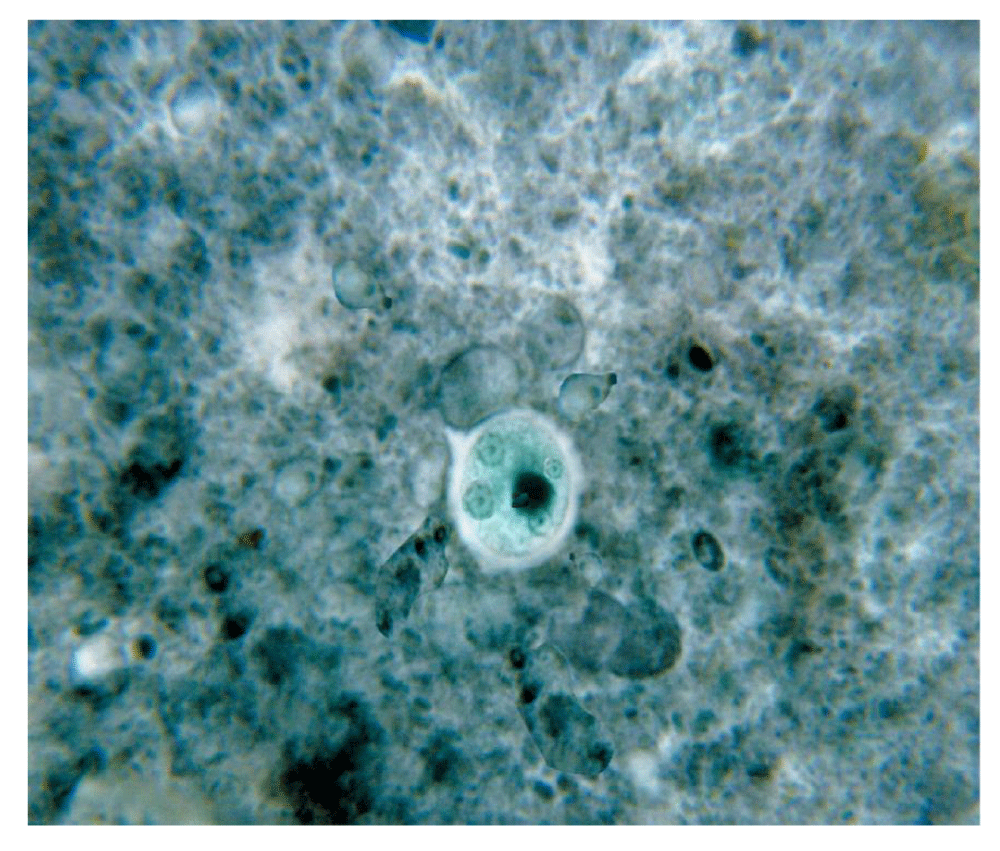
Cysts and trophozoites are seen simultaneously in GSE instances, depending on whether the disease is acute, chronic, or carrier. The Entamoeba histolytica cyst and trophozoites are described in Figure 3.
Additional GSE results included large concentrations of WBCs, RBCs, and monilia when seen using microscopes.
WBCs and eosinophils were slightly increasing, according to CBC examinations. The concentration of platelets and red blood cells rises with time. Elevated creatinine values and high CRP levels were explained by clinical chemistry testing.
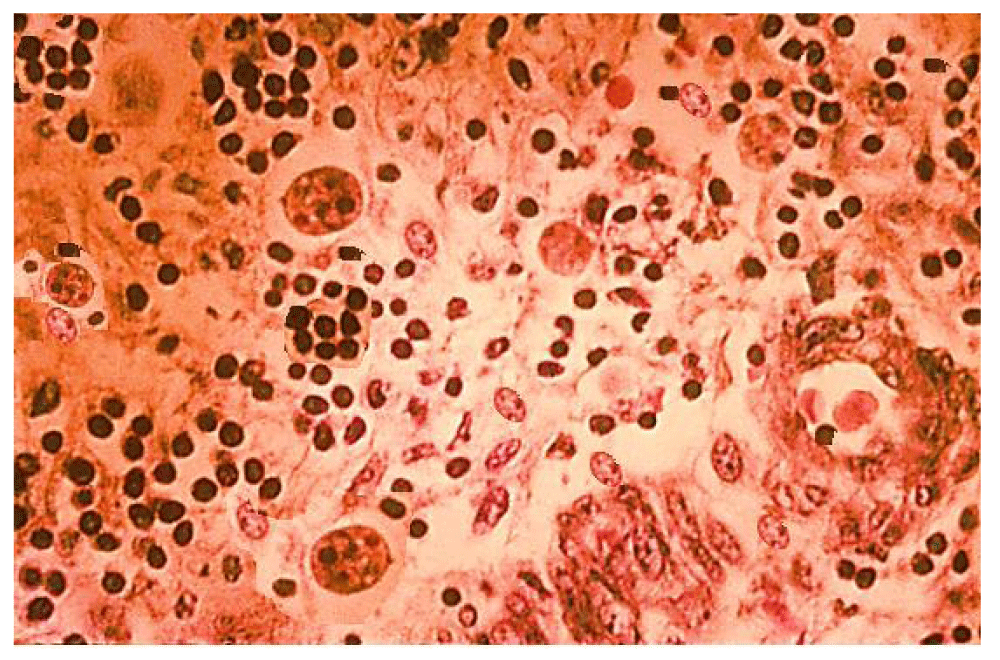
The microscopic analysis of stool samples revealed pus cells and epithelial-tear tissues (Figure 4).
Discussion
The current study evaluated the frequency of intestinal parasites among patients that cause dysentery. This suggests that the prevalence of parasite infection varies geographically. The differences might result from variances in the research population’s characteristics, the geographic dispersion of the population, or the diagnostic methods employed in this and previous investigations [12-14].
Based on the participants’ gender, the prevalence of intestinal protozoa and parasites was evaluated in the current investigation. The findings indicated a noteworthy difference (p < 0.05) in parasite infection cases between research participants who were male and female [15-18].
The results of the biostatic analysis explained why male dysenteric infections were higher than female ones. This could be because men are more independent and spend more time outside, where they are more likely to be infected by parasites. It could also be because men engage in activities like swimming, bird watching, farming, and other hobbies that put them in close contact with potential sources of infection [18-20].
The study also discovered a significant frequency of diarrhea among underweight older persons, with older patients more afflicted than younger ones when comparing dysenteric infection probability (p < 0.05). Given that undernutrition has been linked to diarrhea in the past [21-23], improper nutrition among older people may be one possible reason for this observation.
Once more, research conducted in a remote South Indian village revealed that undernutrition increased the risk of severe diarrhea. Furthermore, environmental as well as personal hygiene are important risk factors for acute diarrhea. Because of their incapacity to take care of themselves and maintain proper personal hygiene, it is conceivable to explain why infections among the elderly have increased [24,25].
There is a notable disparity in the prevalence of diarrhea in rural and urban areas. The likelihood of contracting the illness is higher among those who reside in rural regions. It has been discovered that using unimproved drinking water, having poor sanitation, and having limited access to medical services in rural regions are all positively correlated with a high frequency of diarrhea [25].
Conclusion
Early diagnosis contributes to the identification of the cause of the parasite and thus the choice of the most appropriate treatment. Treatment strategies demonstrate the effectiveness of current treatments and their success in eliminating parasites. This may include assessing the effectiveness of different medicines and comparing different treatment strategies. Prevention and education emphasize the need for health education and prevention of dysentery by improving personal hygiene practices, providing clean water, and reducing environmental pollution. Analysis of the impact of environmental and social factors on the spread of dysentery, such as living conditions and public health behaviors. Future research includes the identification of areas requiring further research, such as the development of new vaccines or more effective and less toxic medicines.
- Marie C, Petri WA. Amoebic dysentery. BMJ Clin Evid. 2013;2013:3758071. PMID: 23991750; PMCID: PMC3758071. Available from: https://pubmed.ncbi.nlm.nih.gov/23991750/
- World Health Organization. Dysentery (Shigellosis) [PDF]. Geneva: World Health Organization; November 2016. p. 2. Archived from the original (PDF) on 2018 Sep 20. Retrieved 2019 Nov 15.
- Shirley DT, Farr L, Watanabe K, Moonah S. A review of the global burden, new diagnostics, and current therapeutics for amebiasis. Open Forum Infect Dis. 2018;5(7). Available from: https://doi.org/10.1093/ofid/ofy161
- Tribble DR. Antibiotic therapy for acute watery diarrhea and dysentery. Mil Med. 2017;182(S2):17-25. Available from: https://doi.org/10.7205/milmed-d-17-00068
- DuPont HL. Interventions in diarrheas of infants and young children. J Am Vet Med Assoc. 1978;173(5 Pt 2):649-653. Available from: https://pubmed.ncbi.nlm.nih.gov/359524/
- DeWitt TG. Acute diarrhea in children. Pediatr Rev. 1989;11(1):6-13. Available from: https://doi.org/10.1542/pir.11-1-6
- Ryan J. Boards and Beyond: Infectious Disease: A Companion Book to the Boards and Beyond Website. 9-26-2016 ed. CreateSpace Independent Publishing Platform. 2016. Available from: https://www.amazon.in/Boards-Beyond-Infectious-Disease-Companion/dp/1523709359
- Salazar-Lindo E, Sack RB, Chea-Woo E, et al. Early treatment with erythromycin of Campylobacter jejuni-associated dysentery in children. J Pediatr. 1986;109:355-360. Available from: https://doi.org/10.1016/s0022-3476(86)80404-8
- Last JM, editor. A dictionary of epidemiology. 3rd ed. New York: Oxford University Press; 1995. Available from: https://www.amazon.com/Dictionary-Epidemiology-Handbooks-Sponsored-IEA/dp/0195096681
- Rivera WI, Tachibana H, Kanbara H. Field study on the distribution of Entamoeba histolytica and Entamoeba dispar in the northern Philippines as detected by PCR. Am J Trop Med Hyg. 1998;59:916-921. Available from: https://doi.org/10.4269/ajtmh.1998.59.916
- Centers for Disease Control and Prevention. Laboratory Methods for the Diagnosis of Epidemic Dysentery and Cholera. Atlanta, GA: Centers for Disease Control and Prevention; 1999. WHO/CDS/CSR/EDC/99.8. Archived from the original (PDF) on 2012 Mar 5.
- Haque R, Faruque ASG, Hahn P, yerly DM, Petri WA Jr. Entamoeba histolytica and Entamoeba dispar infection in children in Bangladesh. J Infect Dis. 1997;175:734-736. Available from: https://doi.org/10.1093/infdis/175.3.734
- Braga LL, Mendonca Y, Paiva CA, Sales A, Cavalcante AL, Mann BJ. Seropositivity for and intestinal colonization with Entamoeba histolytica and Entamoeba dispar in individuals in Northeastern Brazil. J Clin Microbiol. 1998;36:3044-3045. Available from: https://doi.org/10.1128/jcm.36.10.3044-3045.1998
- Chacin-Bonilla L, Bonilla E, Parra AM, Estevez J, Morales LM, Suárez H. Prevalence of Entamoeba histolytica and other intestinal parasites in a community from Maracaibo, Venezuela. Ann Trop Med Parasitol. 1992;86:373-380. Available from: https://doi.org/10.1080/00034983.1992.11812680
- Huston CD, Petri WA. Amoebiasis: clinical implications of the recognition of Entamoeba dispar. Curr Infect Dis Rep. 1999;1:441-447. Available from: https://doi.org/10.1007/s11908-999-0056-9
- Abd-Alla MD, Ravdin JI. Diagnosis of amoebic colitis by antigen capture ELISA in patients presenting with acute diarrhoea in Cairo, Egypt. Trop Med Int Health. 2002;7:365-370. Available from: https://doi.org/10.1046/j.1365-3156.2002.00862.x
- Davis AN, Haque R, Petri WA. Update on protozoan parasites of the intestine. Curr Opin Gastroenterol. 2002;18:10-14. Available from: https://doi.org/10.1097/00001574-200201000-00003
- Lucas R, Upcroft JA. Clinical significance of the redefinition of the agent of amoebiasis. Rev Latinoam Microbiol. 2001;43:183-187. Available from: https://pubmed.ncbi.nlm.nih.gov/17061507/
- Petri WA, Singh U. Diagnosis and management of amebiasis. Clin Infect Dis. 1999;29:1117-1125. Available from: https://doi.org/10.1086/313493
- Stanley SL. Amoebiasis. Lancet. 2003;361:1025-1034. Available from: https://doi.org/10.1016/s0140-6736(03)12830-9
- Haque R, Huston CD, Hughes M, Houpt E, Petri WA Jr. Amebiasis. N Engl J Med. 2003;348:1565-1573. Available from: https://doi.org/10.1056/nejmra022710
- Singh B, Moodley J, Ramdial PK. Fulminant amoebic colitis: a favorable outcome. Int Surg. 2001;86:77-81. Available from: https://pubmed.ncbi.nlm.nih.gov/11918241/
- Petri WA Jr, Mondal D, Peterson KM, Duggal P, Haque R. Association of malnutrition with amebiasis. Nutr Rev. 2009;67(Suppl 2)–15. Available from: https://doi.org/10.1111/j.1753-4887.2009.00242.x
- Ferdous F, Ahmed S, Das SK, Farzana FD, Latham JR, Chisti MJ, Faruque AS. Aetiology and clinical features of dysentery in children aged <5 years in rural Bangladesh. Published online by Cambridge University Press; 2013 Apr 8. Available from: https://doi.org/10.1017%2FS0950268813000666
- Palmer AB. Diarrhoea and Dysentery: Modern Views of Their Pathology and Treatment. Palala Press; 2016. ISBN: 978-1358293467. Available from: https://www.amazon.in/Diarrh-Dysentery-Modern-Pathology-Treatment/dp/1358293465
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
