Archive of Gerontology and Geriatrics Research
Case Report: Extent of the Clinical Spectrum for C9orf72 Mutation - From Frontotemporal Dementia to Autonomic Dysfunction
Catherine Takeda-Raguin1*, Pierre Olivier Lang2, Jérémie Perisse1, Nathalie Philippi3, Patrick Karcher1 and Thomas Vogel1
2Geriatric and Geriatric Rehabilitation Division, Department of Medicine, University Hospital of Lausanne, Lausanne, Switzerland
3Department of Neurology, University Hospitals of Strasbourg, Strasbourg, France
Cite this as
Raguin CT, Lang PO, Perisse J, Philippi N, Karcher P, et al. (2017) Case Report: Extent of the Clinical Spectrum for C9orf72 Mutation - From Frontotemporal Dementia to Autonomic Dysfunction. Peertechz J Gerontol Geriatr Res 2(1): 001-002. DOI: 10.17352/aggr.000004C9ORF72 gene mutation on chromosome 9 corresponding to a repetition of hexanucleotides (GGGGCC) xn is the most common mutation found in frontotemporal lobar dementia (FTLD) and amyotrophic lateral sclerosis (SLA) [1]. FTLD is characterized by an insidious onset with gradual evolution, a decline in social and interpersonal behaviors, self-regulation and control disturbances in personal behavior, emotional blunting and loss of introspection capabilities) but also behavioral disorders, disorders of speech and language.
Literature is controversial about the relationship between multiple system atrophy (MSA) and the gene C9ORF72 mutation. We report the case of a 70-year old patient diagnosed with familial FTLD with C9ORF72 mutation in 2013 associated with cerebellar syndrome, visual hallucinations, and rapidly progressive symptoms suggestive of MSA.
Introduction
Literature is controversial concerning the link between C9orf72 gene mutation and multiple system atrophy (MSA). We report the case of a 70-year old woman diagnosed with familial FTLD with C9orf72 mutation who secondarily developed symptoms related to MSA during her hospitalization.
Case Report
At the time of her first consultation (2012), this patient presented anterograde amnesia and was consecutively diagnosed with mild Alzheimer’s disease (AD). Subsequently, she developed behavioral syndrome suggestive of a frontal syndrome (apathy, desinhibition, gluttony…) and anosognosia. Physical examination revealed mild Parkinsonism and cerebellar syndrome. There was no history of alcohol abuse. Her background history revealed hypertension, obliterative arteriopathy of the lower limbs, sleep apnea syndrome, and gastroesophageal reflux disease. In 2010, she was diagnosed with a frontal-temporal lobular dementia (LDL) with a cognitive disorder associated with antero-retrograde amnesia that initially suggested the diagnosis of Alzheimer’s disease (AD) Motivating anticholinesterase therapy, temporo-spatial disorientation, language disorders, and dyspecific syndrome (see neuropsychological evaluation below).
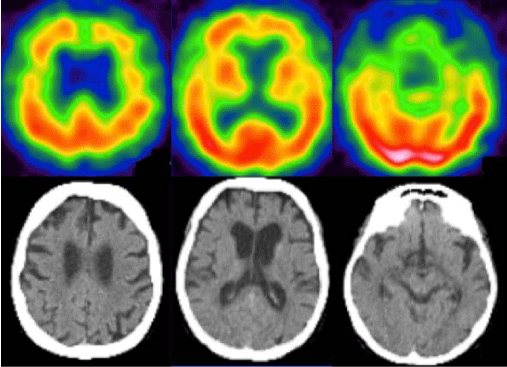
Anamnestic revealed visual illusions and hallucinations without fluctuations or dysautonomic symptoms. The Mini Mental State Examination (MMSE) scored 25/30 (-3 points for orientation; -1 point for attention and language) and the neuropsychological profile suggested frontal lobar dysfunction. Structural and functional imaging by CT-scan and SPECT-scan demonstrated atrophy and cerebral cortical hypoperfusion prominently within frontotemporal regions and slightly asymmetric in favor of the left side (Figure 1). Standard blood and cerebrospinal fluid analyses (protein MA – protein Aβ1-42: 1335 pg/ml – normal; Tau protein: 451 pg/ml – normal; Phospho-Tau protein: normal 57 pg/ml) were normal. Together, these findings led to the diagnosis of Fronto-temporal lobar disease (FTLD). Genetic testing was proposed because of a familial history of dementia associated with behavioral changes for her mother and older sister and a pathologic expansion was found in C9orf72 (2013), confirming a behavioral variant of FTLD.
Over a 2-year period, she severely deteriorated from cognitive, behavioral and functional points of view and was admitted into a nursing home. Upon admission, physical examination revealed a bilateral and symmetrical extrapyramidal hypertonia without tremulation and fasciculation, a spontaneous mouth opening with possible closure. Swallowing capacity was severely compromised. A week later, she suddenly presented severe hypotension, tachycardia, hypothermia and polypnea with a saturation of 94% in room air. The patient was oliguric and had a generalized flaccid hypotonia. Capillary blood glucose was 1.6 g / l (8.9 mmol / L) and the electrocardiogram found sinus tachycardia without an electrocardiographic and gasometric argument for pulmonary embolism. Because of swallowing disorders and sacral pressure ulcer, severe sepsis was suspected and broad spectrum antibiotics started (piperacillin/tazobactam). She rapidly recovered; biological and bacteriological analyses excluded acute infection. Antibiotics were suspended and the clinical situation remained stable. A few weeks later, a similar episode of hypotension, hypothermia and mottling area occurred with bradycardia. No specific etiology was found, so no specific treatment was initiated. During the last two weeks of her life, multiple subsequent vegetative episodes occurred, alternating profuse sweating, isolated hypothermia, lability of blood pressure, paroxysmal tachycardia access and an isolated and temporary mottling. Unfortunately, post-mortem brain analysis was refused by relatives.
Discussion
FTLD is characterized by degeneration of frontal and temporal lobes associated with progressive and dramatic personality, behavior and language changes [2]. In 2011, the autosomal dominant mutation of C9orfF72 gene on the 9p21 chromosome corresponding to a repetition of hexanucleotides (GGGGCC) xn was discovered in family forms of FTLD and ALS [1]. MSA is a rare neurodegenerative disease usually diagnosed after age 50 and characterized by Parkinsonism, ataxia and autonomic dysfunction [3]. Post-mortem analysis reveals areas of degeneration with alpha-synuclein inclusions in oligodendrocytes and neuronal loss at the olivopontocerebellar level and in the nigrostriatal system [4]. Compared to what was initially suspected, the etiologic spectrum of C9orf72 mutation appears not to be limited to FTLD [1]. The association with ALS is well recognized, and the mutation could encompass other Parkinson-like syndromes [5], including MSA, progressive supranuclear paralysis and corticobasal degeneration [6] and Lewy body dementia [7], episodic memory disorder may be associated with forms of Alzheimer’s disease [8].
Conclusion
In this case, family FTLD was diagnosed by typical clinical presentation and genetic confirmation. The presence of severe autonomic disorder with static and kinetic cerebellar syndrome illustrates the wide clinical spectrum of the C9orf72 mutation. Clinical MSA was recently reported with this mutation in a few cases [9]. Unfortunately, none of these cases were confirmed as typical MSA by neurohistology and C9orf72 mutation was never found in any typical case of MSA confirmed by autopsy [10]. Our hypothesis of a link between MSA and the C9orf72 mutation requires analysis of brain tissue.
Author contributions
(1) The conception and design of the study, or acquisition of data, or analysis and interpretation of data: Takeda-Raguin, Philippi, Karcher and Vogel;
(2) Drafting the article or revising it critically for important intellectual content: Takeda-Raguin, Lang, Philippi, Perisse, Karcher and Vogel;
(3) Final approval of the version to be submitted: Takeda-Raguin, Lang, Philippi, Perisse, Karcher and Vogel.
- DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, et al. (2011) Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 72: 245–256. Link: https://goo.gl/TWvJ0u
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, et al. (2011) Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain J Neurol 134: 2456–2477. Link: https://goo.gl/6Gn3hy
- Low PA, Reich SG, Jankovic J, Shults CW, Stern MB, et al. (2015) Natural history of multiple system atrophy in the USA: a prospective cohort study. Lancet Neurol 14: 710–719. Link: https://goo.gl/1ZkWv2
- Ahmed Z, Asi YT, Sailer A, Lees AJ, Houlden H, et al. (2012) The neuropathology, pathophysiology and genetics of multiple system atrophy. Neuropathol Appl Neurobiol 38: 4–24. Link: https://goo.gl/aQhjWD
- Schottlaender LV, Holton JL, Houlden H (2014) Multiple system atrophy and repeat expansions in C9orf72. JAMA Neurol 71: 1190–1191. Link: https://goo.gl/v9xGJh
- Lindquist SG, Duno M, Batbayli M, Puschmann A, Braendgaard H, et al. (2013) Corticobasal and ataxia syndromes widen the spectrum of C9ORF72 hexanucleotide expansion disease. Clin Genet 83: 279–283. Link: https://goo.gl/V7DL4S
- Snowden JS, Rollinson S, Lafon C, Harris J, Thompson J, et al. (2012) Psychosis, C9ORF72 and dementia with Lewy bodies. J Neurol Neurosurg Psychiatry 83: 1031–1032. Link: https://goo.gl/NLRbTQ
- Davidson YS, Robinson AC, Snowden JS, Mann DMA (2013) Pathological assessments for the presence of hexanucleotide repeat expansions in C9ORF72 in Alzheimer’s disease. Acta Neuropathol Commun 1: 50. Link: https://goo.gl/vDBvVJ
- Goldman JS, Quinzii C, Dunning-Broadbent J, Waters C, Mitsumoto H, et al. (2014) Multiple system atrophy and amyotrophic lateral sclerosis in a family with hexanucleotide repeat expansions in C9orf72. JAMA Neurol 71: 771–774. Link: https://goo.gl/Ytu2CJ
- Scholz SW, Majounie E, Revesz T, Holton JL, Okun MS, et al. (2015) Multiple system atrophy is not caused by C9orf72 hexanucleotide repeat expansions. Neurobiol Aging 36: 1223.e1–2. Link: https://goo.gl/ViHf7E
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
