Annals of Antivirals and Antiretrovirals
Analysis of baseline laboratory characteristics of HIV-positive patients in Bulgaria treated with bictegravir /emtricitabine/ tenofovir anafelamide (early analysis)
Dimitar Strashimirov*, Ralitsa Yordanova, Rusina Grozdeva, Evgeni Penchev, Daniel Ivanov and Nina Yancheva-Petrova
Cite this as
Strashimirov D, Yordanova R, Grozdeva R, Penchev E, Ivanov D, et al. (2024) Analysis of baseline laboratory characteristics of HIV-positive patients in Bulgaria treated with bictegravir /emtricitabine/ tenofovir anafelamide (early analysis). Ann Antivir Antiretrovir 8(1): 001-007. DOI: 10.17352/aaa.000018Copyright
© 2024 Strashimirov D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Introduction: Bictegravir/Emtricitabine/Tenofovir Anafelamide (B/F/TAF) is a recommended single tablet regimen with good tolerability and safety as well as durability of the virologic response. From its introduction in the country of Bulgaria in April 2021 till November 2021 it was given to 82 patients, followed up and treated in the Department for HIV at the Prof. Ivan Kirov Specialized Hospital for Active Treatment of Infectious and Parasitic Diseases, in 60 (72%) of which the therapeutic regimen was changed mainly from protease – inhibitor-containing regimens.
Materials and methods: We present the initial 24-week period of the application of this therapeutic regimen. Our purpose was to analyze the main reasons for a switch of the cART among patients, in which the therapeutic regimen was switched, to analyse the dynamics of the basic immunologic and virologic values of the patients, and to try to analyse the safety and efficacy of the regimen.
Results: Patients of nearly all age groups were enrolled in the study. The main therapeutic regimens, from which patients were switched to B/F/TAF were protease inhibitor-based, the main reason being suboptimal viral suppression, followed by simplification of cART. Side effects were the third leading cause for the switch, mainly due to gastrointestinal symptoms. Nearly half of the patients had CD 4+ T-cell count of > 500/mm3 at the time of the switch and undetectable viral load levels. The test statistical analyses of the immunological and virologic levels before and after the administration of B/F/TAF showed statistical significance as far as the viral load was concerned. Analyses of factors of prognostic importance for maintaining stable virologic response showed that CD + T-cell values, CD4:CD8 ratio before administration, VL before administration as well as time of intake proved to be of prognostic value. Cholesterol levels in patients with hypercholesterolemia were significantly lower.
Conclusion: A relatively large number of patients were enrolled for a relatively short period of time; B/F/TAF proved to be of good efficacy and safety.
Abbreviations
ABC/3TC: Abacavir and Lamivudine; ABC/3TC/DTG: Abacavir, Lamivudine, Dolutegravir; ATZ/r: Atazanavir and Ritonavir; ALAT: Alanine Aminotransferase; DRV/c: Darunavir Boosted with Cobicistat; B/F/TAF: Bitegravir/Emtricitabine/Tenofovir Anafelamide; DRV/r: Darunavir, Boosted with Ritonavir; ETR: Etravirine; FTC/TDF/DTG: Emtricitabine, Tenofovir; Dolutegravir; TAF/FTC/EVG/c: Tenofovir Anafelamide; Emtricitabine; Elvitegravir, Cobicistat; LPV/r: Liponavir and Ritonavir; RAL: Raltegravir; cART: combined Antiretroviral Therapy; VL: Viral Load; INSTI: Integrase Strand Transfer Inhibitor
Introduction
The use of multiple drugs that act on different viral targets is known as combined Antiretroviral Therapy (cART). Since its introduction it has become the cornerstone of the treatment of HIV infection; cART decreases the patient’s total burden of HIV, maintains the function of the immune system, and prevents opportunistic infections that often lead to death [1,2]. The introduction of novel antiretroviral medications has been challenged as far as safety and efficacy are concerned, i.e. better tolerance, fewer side effects, less drug-to-drug interactions, stable immunologic and virologic response, time for achieving optimal viral suppression (VL < 50 copies/ml). Integrase strand transfer inhibitors are recommended in initial antiretroviral therapy for HIV-1 infection according to multiple guidelines [2-6]. The combination regimen that contains three antiviral agents: Emtricitabine, Tenofovir anafelamide, and Bictegravir (B/F/TAF) may be used to treat HIV-1 in adults and children weighing more than 25kg (55lb). It is generally well-tolerated and the most common side effects reported are diarrhea, nausea, and headache are rarely reported [7-14]. Research has shown that after 48 weeks B/F/TAF reduced HIV-1 viral load to <50 copies/mL in over 92% of patients and to< 20 copies/mL in over 87% of people [15-18]. The therapeutic regimen was beneficial in cases of HIV/HBV coinfection (due to the restricted distribution of Tenofovir anafelamide in cells with high carboxylase and cathepsin A activity, resulting in the higher distribution in hepatocytes and lymphocytes) as well as more favourable renal and bone safety [19-21]. Studies showed durability against M184V/s and pre-existing INSTI resistance, as well as patients with historical virological failures and K65N/R mutation [22-24]. Another characteristic feature is rapid viral suppression [25-27]. B/F/TAF has been available in our country, Bulgaria since April 2021. We focused on analyses of the basic characteristics of patients in which B/F/TAF was given as a therapeutic regimen, to analyse the reasons for the switch of the therapy regarding patients, in which the therapeutic regimen was changed, to analyse the dynamics of the basic immunologic and virologic characteristics of the patients, to try to analyse the safety and efficacy of the regimen.
Materials and methods
This is an observational retrospective real-life cohort study, which enrolled all patients in which Bictegravir-containing regimen – B/F/TAF was given either as an initial treatment option or the patients were switched from another therapeutic regimen, which we described as well as the reasons for the switch. We analyzed the initial 24-week period after the administration of the medication. Patients were followed up in 4 – 16 weeks according to the viral load levels, patients with undetectable viral load levels were followed up in 8 weeks until viral load dropped to undetectable; those with undetectable viral load levels were followed up in 16 weeks period. On follow-up visits basic laboratory characteristics were examined, among which we analysed the CD 4 + and CD8 + T-cell counts, viral load, and basic biochemical values, among which we focused on lipid profile analysis. Afterward, we tried to find correlations between the immunological values before and after the switch, as well as the time of intake of the medication and tried to look for prognostic values as far as maintaining undetectable viral load levels was concerned. Statistical analysis was performed by means of descriptive analysis, scatterplot, nonparametric tests (ANOVA), Kolmogorov – Smirnov test, t-tests, nonparametric testing of correlations) and binary logistic regression. The F – and p – values were obtained by means of ANOVA and T-tests. The study was conducted after approval of the Ethical Committee of The Ivan Kirov Specialized Hospital for Infectious and Parasitic Diseases (Approval Protocol P-4/2021). Informed consent was obtained from all the patients.
Results
The average age of the patients was 42 ± 11 years in the range of 16-71 years. The distribution of the patients according to age groups is shown in Table 1. There was a prevalence of the age group 40-50 years, followed by 30-40 years, groups 20-30 and > 50 years were presented with equal percentage, and only one patient was below 20 years of age (16 years).
The distribution according to previous regimens of the cART, initiation, or continuation of already initiated therapy are shown in Table 2.
B/F/TAF was used as a first-line regimen for cART initiation in 17 patients (20.7%). The main cART regimens, from which patients were switched to B/F/TAF were protease inhibitors based in 19 cases (29.2%), from which 11 patients were receiving Darunavir: 8 boosted with Cobicistat /DRV/c/, 3 - with Ritonavir /DRV/r/. Five patients were receiving the LPV/r regimen and 3 with - ATZ/r. The main regimen, from which patients were switched to B/F/TAF was ABC/3TC/DTG, followed by FTC/TDF/DTG in 9 (11%). The reason for switching a patient receiving the combination TAF/FTC/EVG/c was the lack of the corresponding medications or the single tablet form in Bulgaria.
It is obvious that suboptimal viral suppression is the leading cause for the switch to B/F/TAF, followed by simplification of cART. Side effects were the third leading cause for the switch of the therapy (Table 3). The main side effects included gastrointestinal symptoms - nausea and diarrhea among patients, receiving protease inhibitor-based regimens and neurologic complications (insomnia, anxiety) among patients, receiving integrase inhibitor-based regimens, mainly Dolutegravir.
Afterward, we focused on analysing the main immunologic parameters of the patients by using descriptive statistics. The levels of CD 4+, CD 8+, and CD4:CD8 ratio before the introduction of B/F/TAF and starting follow-up analysed in our department are shown in Table 4. It is seen that the mean CD 4+ T-cell counts were above 450/mm3.
Subsequently, we analyzed the patients by splitting their CD 4+ T-cell counts into groups: first group below 200 cells/mm3, second - 200-500 cells/mm3, and third - above 500 cells/mm3 and below and above 350/mm3. Results are shown in Tables 5,6.
Viral load levels before the introduction of B/F/TAF are shown in Table 7. Viral load levels and immunologic characteristics of the patients after the introduction of B/F/TAF are shown in Tables 8,9.
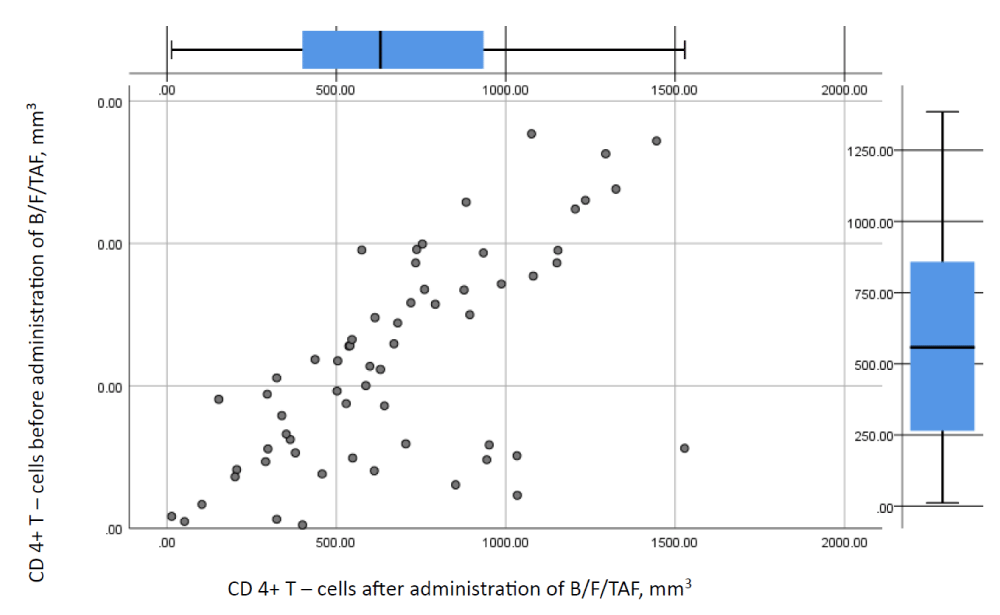
Thereafter we made an attempt to compare the immunologic values of the patients by means of test statistics. Results of the analysis are shown in Figure 1 and Table 10. No statistically significant differences in the CD 4+T-cell counts between the groups were found (level of rejection of the null hypothesis set at p < 0.05).
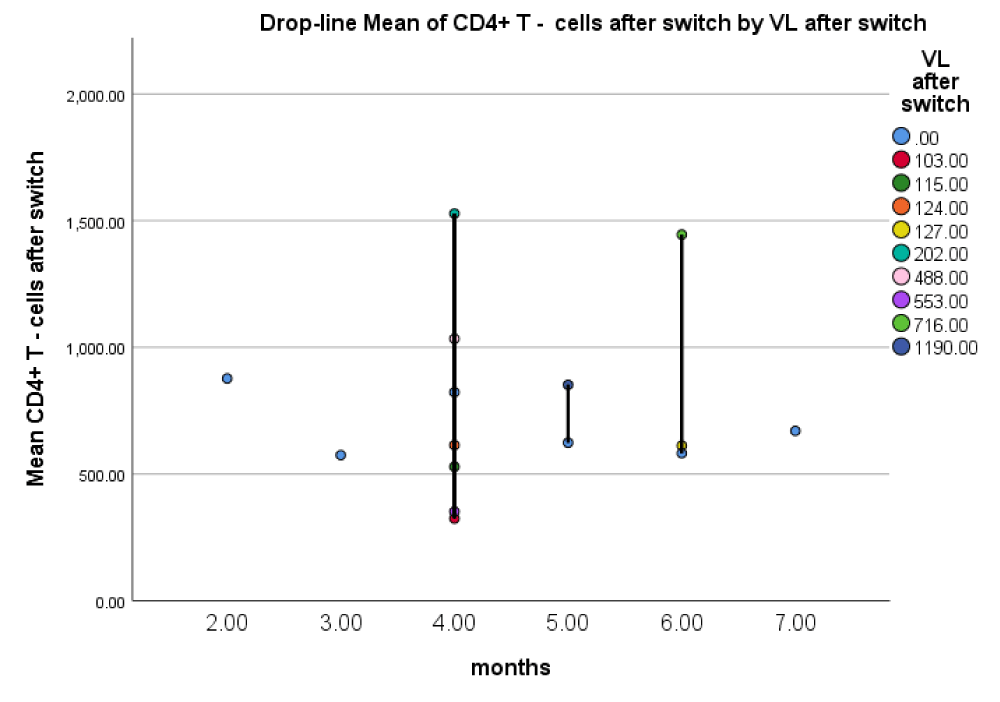
However, analysis of the virologic data showed a statistically significant difference (Figure 2, Table 11). The time of intake of the medication was an average of 17 weeks (16.79 ± 6.11 weeks in the range of 4 – 28 weeks). The nonparametric testing of correlations between time of intake, immunologic and virologic characteristics of the patients however showed no statistical significance (Figures 3, Table 12).
Most of the patients were clustered at 4 months (16 weeks), as the majority of them had CD4+ T-cell counts > 500 cells/µcl and mainly undetectable VL levels after 6 months (24 weeks).
We tried to find out prognostic factors for optimal viral suppression (VL < 50 c/ml) 4-24 weeks after administration of B/F/TAF. For this purpose, the following parameters were tested using binary logistic regression analysis:
CD 4+ T-cells before administration
- CD4:CD8 ratio before administration
- VL before administration
- CD4 + T-cell count after administration
- CD4:CD8 ratio after administration
Statistical significance was found and the parameters proved to be of prognostic importance (p = 0.000). The following values proved to be of statistically significant prognostic importance (p = 0.04): CD 4+ T-cell count, VL before administration of B/F/TAF, as well as the time of intake of the medication.
Patients, in which the therapeutic regimen was switched to B/F/TAF due to dyslipidaemia were only 6, and data for patients are shown in table 13. Paired sample statistics were performed for the groups before and after administration of B/F/TAF and statistical significance was found for cholesterol levels (p = 0.016).
Discussion
As a last-generation therapeutic regimen, B/F/TAF is а first line choice without alternatives in certain cases: dyslipidemia, concomitant intake of certain medications with multiple drug-drug interactions, and underlying diseases [7,8,12,17]. It is the medication of choice for rapid start of antiretroviral therapy even on the same day of the registration; timing might be tight and the ability of on-time action is of significant importance. It is a regimen of choice for the switch of antiretroviral therapy especially when the patient experiences side effects of his contemporary regimen, which might be excruciating and could affect the adherence of the patient to the therapy. A high barrier of virologic resistance makes the drug suitable for switch of the therapeutic regimen for patients with inadequate virologic response [7,10,13,14,17,18,20,28- 31].
We analyzed data for 82 patients in which B/F/TAF was introduced as a therapeutic regimen, 91% of which were male. Patients were relatively young (42 ± 11 years of age), however the main group was 41 – 50 years (36%). 17% were above 50 years of age. Aging with HIV is an important cornerstone of contemporary HIV medicine and the introduction of novel medications with fewer side effects, DDIs, and maintaining durable immunologic and virologic response is of significant importance.
In 21% of the patients B/F/TAF was used for initiation of cART. In 4 patients ART was initiated on the day of their first visit and registration. From those patients, in which the therapeutic regimen was changed, most were receiving protease inhibitors, mainly Darunavir, and the main integrase inhibitor-based regimen, that was changed to B/F/TAF was Dolutegravir. Considering the reasons for the switch, we should point out suboptimal viral suppression as the leading cause in all regimens, from which patients were switched, mainly protease inhibitor-based regimens. The next reason for changing the therapeutic regimen was the simplification of cART. Intake of one pill daily without dependence on food intake is of great benefit and preferred by patients compared to several pills or two times daily regimens, as well as medications whose intake should comply with food intake [6,7,10]. Nephrologic complications necessitate the exclusion of Tenofovir disoproxil fumarate /TDF/ containing regimens and a switch to Tenofovor anafelamide /TAF/. Regimens, containing Abacavir should not be considered in cases of cardiac diseases. These patients are often dyslipidemic and are treated with cardiotonic medications with multiple DDIs, a fact, that makes them inappropriate for treatment with protease-inhibitor-based regimens. Analogically Dolutegravir-based regimens are inappropriate in case of neurologic complaints, as well as sleep disturbances or insomnia, and B/F/TAF showed noninferiority [11,31]. Raltegravir should be excluded when the patient experiences muscle pains [14,18].
Before the application of B/F/TAF majority of the patients had CD 4+ T-cell count > 350/mm3 – the critical CD 4+ T-cell count, which puts them at risk for the development of opportunistic infections. Nearly half of them /48.8 percent/ had CD 4+ T-cell count above 500/mm3. Similarly, nearly half of them had undetectable viral load levels. After the switch patients had an average CD 4+ above 500/mm3, thus maintaining good immunologic parameters. However, analyses showed no statistical significance between CD 4+ T-cell count values before and after administration.
Analyses of virologic parameters showed significant differences in the viral load levels after the introduction of the medication. The rapid drop to undetectable values within 8 weeks was observed in more than half of the patients, in which B/F/TAF was initiated as a first-line treatment option. Patients with undetectable viral load levels before the switch showed durability as far as virological response was concerned. In opposite to the immunologic parameters, here analyses revealed statistically significant differences.
Simultaneous testing of CD 4+ T-cell and viral load values showed optimal immunologic (CD 4+ T-cell count in the range 500 – 1500 cells/mm3) with undetectable viral load levels 4 months (16 weeks) after the switch.
All values, that were tested, proved to be of prognostic importance for maintaining stable undetectable VL levels 4-16 weeks after application of Bictegravir.
Patients, who were switched due to dyslipidemia showed good response with cholesterol levels significantly lower after the switch.
Conclusion
After initiation or switch to a Bictegravir-containing regimen, no new side effects were observed. As far as the switch of the cART was concerned patients from all possible therapeutic regimens were switched. A stable virologic response was observed. As far as the immunologic response was concerned, no statistical significance in the immunologic parameters was proved. The analyses of viral load levels, however, showed the statistical significance of the values after the switch. Satisfactory results from the lipid profile were also found, with cholesterol levels after the switch being significantly lower. We should point out the relatively large number of patients enrolled for a relatively short period of time in the country and nearly excellent results for a short period of follow-up by means of immunologic and virologic response.
- Aberg JA, Gallant JE, Ghanem KG, Emmanuel P, Zingman BS, Horberg MA; Infectious Diseases Society of America. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV medicine association of the Infectious Diseases Society of America. Clin Infect Dis. 2014 Jan;58(1):e1-34. doi: 10.1093/cid/cit665. Epub 2013 Nov 13. PMID: 24235263.
- Scarsi KK, Havens JP, Podany AT, Avedissian SN, Fletcher CV. HIV-1 Integrase Inhibitors: A Comparative Review of Efficacy and Safety. Drugs. 2020 Nov;80(16):1649-1676. doi: 10.1007/s40265-020-01379-9. PMID: 32860583; PMCID: PMC7572875.
- Micán R, de Gea Grela A, Cadiñanos J, de Miguel R, Busca C, Bernardino JI, Valencia E, Montes ML, Montejano R, Moreno V, Pérez Valero I, Serrano L, González-García J, Arribas JR, Martín-Carbonero L. Impact of preexisting nucleos(t)ide reverse transcriptase inhibitor resistance on the effectiveness of bictegravir/emtricitabine/tenofovir alafenamide in treatment experience patients. AIDS. 2022 Nov 15;36(14):1941-1947. doi: 10.1097/QAD.0000000000003311. Epub 2022 Jul 8. PMID: 35848506; PMCID: PMC9612675.
- Ryom L, De Miguel R, Cotter AG, Podlekareva D, Beguelin C, Waalewijn H, Arribas JR, Mallon PWG, Marzolini C, Kirk O, Bamford A, Rauch A, Molina JM, Kowalska JD, Guaraldi G, Winston A, Boesecke C, Cinque P, Welch S, Collins S, Behrens GMN; EACS Governing Board. Major revision version 11.0 of the European AIDS Clinical Society Guidelines 2021. HIV Med. 2022 Sep;23(8):849-858. doi: 10.1111/hiv.13268. Epub 2022 Mar 25. PMID: 35338549; PMCID: PMC9545286.
- Wohl DA, Yazdanpanah Y, Baumgarten A, Clarke A, Thompson MA, Brinson C, Hagins D, Ramgopal MN, Antinori A, Wei X, Acosta R, Collins SE, Brainard D, Martin H. Bictegravir combined with emtricitabine and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection: week 96 results from a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet HIV. 2019 Jun;6(6):e355-e363. doi: 10.1016/S2352-3018(19)30077-3. Epub 2019 May 5. PMID: 31068270.
- Gandhi RT, Bedimo R, Hoy JF, Landovitz RJ, Smith DM, Eaton EF, Lehmann C, Springer SA, Sax PE, Thompson MA, Benson CA, Buchbinder SP, Del Rio C, Eron JJ Jr, Günthard HF, Molina JM, Jacobsen DM, Saag MS. Antiretroviral Drugs for Treatment and Prevention of HIV Infection in Adults: 2022 Recommendations of the International Antiviral Society-USA Panel. JAMA. 2023 Jan 3;329(1):63-84.
- Ambrosioni J, Rojas Liévano J, Berrocal L, Inciarte A, de la Mora L, González-Cordón A, Martínez-Rebollar M, Laguno M, Torres B, Ugarte A, Chivite I, Leal L, de Lazzari E, Miró JM, Blanco JL, Martinez E, Mallolas J. Real-life experience with bictegravir/emtricitabine/tenofovir alafenamide in a large reference clinical centre. J Antimicrob Chemother. 2022 Mar 31;77(4):1133-1139. doi: 10.1093/jac/dkab481. PMID: 35040990.
- Avihingsanon A, Lu H, Leong CL, Hung CC, Koenig E, Kiertiburanakul S, Lee MP, Supparatpinyo K, Zhang F, Rahman S, D'Antoni ML, Wang H, Hindman JT, Martin H, Baeten JM, Li T; ALLIANCE Study Team. Bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir, emtricitabine, and tenofovir disoproxil fumarate for initial treatment of HIV-1 and hepatitis B coinfection (ALLIANCE): a double-blind, multicentre, randomised controlled, phase 3 non-inferiority trial. Lancet HIV. 2023 Oct;10(10):e640-e652.
- Baldin G, Ciccullo A, Lombardi F, D'Angelillo A, Dusina A, Emiliozzi A, Farinacci D, Moschese D, Picarelli C, Borghetti A, Di Giambenedetto S. Short Communication: Comparing Lamivudine+Dolutegravir and Bictegravir/Emtricitabine/Tenofovir Alafenamide as Switch Strategies: Preliminary Results from Clinical Practice. AIDS Res Hum Retroviruses. 2021 Jun;37(6):429-432. doi: 10.1089/AID.2020.0219. Epub 2020 Dec 30. PMID: 33280486.
- Jain P, Morrisette T, Scherrer S. Pharmacist-led patient satisfaction survey for people living with human immunodeficiency virus taking single-tablet bictegravir/emtricitabine/tenofovir alafenamide after being switched from two tablet dolutegravir plus emtricitabine/tenofovir alafenamide. In: Proceedings of the 2019 ACCP Annual Meeting, New York, 26-29 October 2019.
- Jing C, Wei T, Ning W, Fang Z, Gang X, Xingzhi W, Guoqiang Z, Min W. Treatment persistence of bictegravir/emtricitabine/tenofovir alafenamide and efavirenz + lamivudine + tenofovir disoproxil among HIV-1 patients newly starting treatment in Hunan Province in China. BMC Infect Dis. 2023 Jun 12;23(1):396. doi: 10.1186/s12879-023-08359-w. PMID: 37308847; PMCID: PMC10258991.
- Havens JP, Bares SH, Lyden E, Podany AT, Scarsi KK, Fadul N, Swindells S. Effectiveness and Safety of Bictegravir/Emtricitabine/Tenofovir Alafenamide in Patients With HIV-1 Infection and Ongoing Substance Use Disorder: The BASE Study. Open Forum Infect Dis. 2023 Feb 12;10(3):ofad080. doi: 10.1093/ofid/ofad080. PMID: 36910693; PMCID: PMC10003752.
- Peters E, Iwuji AC. Efficacy, safety and tolerability of Biktarvy in HIV-1 infection: A scoping review. Antivir Ther. 2023 Feb;28(1):13596535231159030. doi: 10.1177/13596535231159030. PMID: 36802921.
- Zhao AV, Crutchley RD, Guduru RC, Ton K, Lam T, Min AC. A clinical review of HIV integrase strand transfer inhibitors (INSTIs) for the prevention and treatment of HIV-1 infection. Retrovirology. 2022 Oct 22;19(1):22. doi: 10.1186/s12977-022-00608-1. PMID: 36273165; PMCID: PMC9588231.
- Dufour I, Fougère Y, Goetghebuer T, Hainaut M, Mbiya B, Kakkar F, Yombi JC, Van der Linden D. Gen Z and HIV-Strategies for Optimizing the Care of the Next Generation of Adolescents Living with HIV. Viruses. 2023 Sep 29;15(10):2023. doi: 10.3390/v15102023. PMID: 37896800; PMCID: PMC10611287.
- Gupta SK, Post FA, Arribas JR, Eron JJ Jr, Wohl DA, Clarke AE, Sax PE, Stellbrink HJ, Esser S, Pozniak AL, Podzamczer D, Waters L, Orkin C, Rockstroh JK, Mudrikova T, Negredo E, Elion RA, Guo S, Zhong L, Carter C, Martin H, Brainard D, SenGupta D, Das M. Renal safety of tenofovir alafenamide vs. tenofovir disoproxil fumarate: a pooled analysis of 26 clinical trials. AIDS. 2019 Jul 15;33(9):1455-1465. doi: 10.1097/QAD.0000000000002223. PMID: 30932951; PMCID: PMC6635043.
- Gallant J, Lazzarin A, Mills A, Orkin C, Podzamczer D, Tebas P, Girard PM, Brar I, Daar ES, Wohl D, Rockstroh J, Wei X, Custodio J, White K, Martin H, Cheng A, Quirk E. Bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection (GS-US-380-1489): a double-blind, multicentre, phase 3, randomised controlled non-inferiority trial. Lancet. 2017 Nov 4;390(10107):2063-2072. doi: 10.1016/S0140-6736(17)32299-7. Epub 2017 Aug 31. PMID: 28867497.
- Gill MSA, Hassan SS, Ahemad N. Evolution of HIV-1 reverse transcriptase and integrase dual inhibitors: Recent advances and developments. Eur J Med Chem. 2019 Oct 1;179:423-448. doi: 10.1016/j.ejmech.2019.06.058. Epub 2019 Jun 24. PMID: 31265935.
- Agarwal K, Brunetto M, Seto WK, Lim YS, Fung S, Marcellin P, Ahn SH, Izumi N, Chuang WL, Bae H, Sharma M, Janssen HLA, Pan CQ, Çelen MK, Furusyo N, Shalimar D, Yoon KT, Trinh H, Flaherty JF, Gaggar A, Lau AH, Cathcart AL, Lin L, Bhardwaj N, Suri V, Mani Subramanian G, Gane EJ, Buti M, Chan HLY; GS-US-320-0110; GS-US-320-0108 Investigators. 96 weeks treatment of tenofovir alafenamide vs. tenofovir disoproxil fumarate for hepatitis B virus infection. J Hepatol. 2018 Apr;68(4):672-681. doi: 10.1016/j.jhep.2017.11.039. Epub 2018 Jan 17. PMID: 29756595.
- Maggiolo F, Rizzardini G, Molina JM, Pulido F, De Wit S, Vandekerckhove L, Berenguer J, D'Antoni ML, Blair C, Chuck SK, Piontkowsky D, Martin H, Haubrich R, McNicholl IR, Gallant J. Bictegravir/Emtricitabine/Tenofovir Alafenamide in Virologically Suppressed People with HIV Aged ≥ 65 Years: Week 48 Results of a Phase 3b, Open-Label Trial. Infect Dis Ther. 2021 Jun;10(2):775-788.
- Gan L, Xie X, Fu Y, Yang X, Ma S, Kong L, Song C, Song Y, Ren T, Long H. Bictegravir/Emtricitabine/Tenofovir Alafenamide Versus Dolutegravir Plus Lamivudine for Switch Therapy in Patients with HIV-1 Infection: A Real-World Cohort Study. Infect Dis Ther. 2023 Nov;12(11):2581-2593. doi: 10.1007/s40121-023-00879-x. Epub 2023 Oct 16. PMID: 37845566; PMCID: PMC10651567.
- Januszka JE, Drwiega EN, Badowski ME. Bictegravir/Emtricitabine/Tenofovir Alafenamide for HIV-1: What is the Hidden Potential of This Emerging Treatment? HIV AIDS (Auckl). 2023 Nov 29;15:705-711. doi: 10.2147/HIV.S385877. PMID: 38050483; PMCID: PMC10693755.
- Sax PE, Rockstroh JK, Luetkemeyer AF, Yazdanpanah Y, Ward D, Trottier B, et al. Switching to bictegravir, emtricitabine, and tenofovir alafenamide in virologically suppressed adults with HIV. Clin Infect Dis 2020; 73:ciaa988
- Wohl DA, Yazdanpanah Y, Baumgarten A, Clarke A, Thompson MA, Brinson C, Hagins D, Ramgopal MN, Antinori A, Wei X, Acosta R, Collins SE, Brainard D, Martin H. Bictegravir combined with emtricitabine and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection: week 96 results from a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet HIV. 2019 Jun;6(6):e355-e363. doi: 10.1016/S2352-3018(19)30077-3. Epub 2019 May 5. PMID: 31068270.
- Balcı U, Üser Ü, Tahmaz A, Sarigul Yildirim F. Real-Life Experience With Bictegravir/Emtricitabine/Tenofovir Alafenamide in Turkey. Cureus. 2023 Oct 18;15(10):e47253. doi: 10.7759/cureus.47253. PMID: 38022124; PMCID: PMC10655161.
- De Clercq E, Zhang Z, Huang J, Zhang M, Li G. Biktarvy for the treatment of HIV infection: Progress and prospects. Biochem Pharmacol. 2023 Nov;217:115862. doi: 10.1016/j.bcp.2023.115862. Epub 2023 Oct 17. PMID: 37858869.
- Tsai MS, Sun HY, Chen CP, Lee CH, Lee CY, Liu CE, Tang HJ, Hung TC, Li CW, Lee YT, Liou BH, Yang CJ, Hung CC; Taiwan HIV Study Group. Switching to co-formulated bictegravir, emtricitabine, and tenofovir alafenamide maintained viral suppression in adults with historical virological failures and K65N/R mutation. Int J Infect Dis. 2023 Jan;126:39-47.
- Hughes DL. Review of synthetic routes and final forms of integrase inhibitors dolutegravir, cabotegravir, and bictegravir. Org Process Res Dev. 2019; 23: 716-729.
- Koneru PC, Francis AC, Deng N, Rebensburg SV, Hoyte AC, Lindenberger J, Adu-Ampratwum D, Larue RC, Wempe MF, Engelman AN, Lyumkis D, Fuchs JR, Levy RM, Melikyan GB, Kvaratskhelia M. HIV-1 integrase tetramers are the antiviral target of pyridine-based allosteric integrase inhibitors. Elife. 2019 May 23;8:e46344.
- Rojas Lievano J, Inciarte A, De La Mora L. Experience from a real-life cohort: Outcome of 984 HIV-infected patients treated with bictegravir/emtricitabine/tenofovir alafenamide in Hospital Clinic, Barcelona. In: Proceedings of the HIV Glasgow 2020, Glasgow, 5-8 October 2020. Bictegravir/emtricitabine/tenofovir alafenamide as initial treatment for HIV-1: five-year follow-up from two randomized trials. EClinicalMedicine. 2023 May 11;59:101991.
- Shafer RW, Vuitton DA. Highly active antiretroviral therapy (HAART) for the treatment of infection with human immunodeficiency virus type 1. Biomed Pharmacother. 1999 Mar;53(2):73-86. doi: 10.1016/s0753-3322(99)80063-8. PMID: 10337461.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.



 Save to Mendeley
Save to Mendeley
