Annals of Antivirals and Antiretrovirals
Castalagin: Some aspects of the mode of Anti-Herpes Virus Activity
N Vilhelmova-Ilieva1, D Deffieux2,S Quideau2, AS Galabov1*
2Univ. Bordeaux, ISM (CNRS-UMR 5255), 351 cours de la Libération, 33405 Talence Cedex, France
Cite this as
Vilhelmova-Ilieva N, Deffieux D, Quideau S, Galabov AS (2018) Castalagin: Some aspects of the mode of Anti-Herpes Virus Activity. Ann Antivir Antiretrovir 2(1): 004-007. DOI: 10.17352/aaa.000004Castalagin, a nonahydroxytriphenoyl-bearing C-glucosidic ellagitannin, manifested a marked virucidal effect on HSV-1. This effect was markedly temperature and time dependent, being clearly higher at 37 °C than at room temperature: Δlog of 3.13 with 10 μM concentration of castalagin (MNC). Castalagin also suppressed HSV-1 particle attachment to MDBK cells. A significant effect (Δlog = 1.7) was found after 30 min of substance exposure and was increased at 60 min (Δlog = 3.2). Castalagin effect on the production of virions during the HSV-1 replication cycle was studied using a time-of-addition experimental scheme at the one-step viral replication cycle design. The highest sensitivity to castalagin was recorded 0–3 h post virus inoculation. A substantially weaker effect was found at the 6–12 h time intervals. At 18–24 h, HSV-1 replication was unsusceptible to castalagin. Evidently, castalagin target is located in the earlier stages of the HSV-1 replication cycle.
Introduction
Herpes simplex virus (HSV) is a DNA-enveloped virus that exists as two types: HSV-1 and HSV-2, of which HSV-1 is mainly associated with mouth disease, and HSV-2 is more commonly associated with genital disease. These cause some of the most common infections in humans and are globally prevalent [1]. After the primary infection, a persistent (latent) life-long infection is established. This infection can be reactivated, causing a recurrent disease condition. The most severe infections are encephalitis in neonates and disseminated infections in patients with defects in cellular immunity [2-4].
Acyclovir (ACV) was the first effective virus-specific anti-herpesvirus drug made available [5]. Since then, other nucleoside analogues have been developed, but their efficacy is often compromised, as is that of ACV, by the appearance of drug-resistant HSV mutants. Therefore, it is critically important to search for novel anti-herpesvirus compounds with non-nucleoside structures and with different mechanisms of action [6-9].
Tannins are a group of plant polyphenols that notably include hydrolyzable and condensed tannins. Hydrolyzable tannins, such as ellagitannins and gallotannins, can be hydrolyzed into glucose, gallic and ellagic acids [10-13]. Ellagitannins are potential inhibitors of various enveloped viruses due to their ability to bind with different proteins, thus altering the proteins structure and inactivating them [14-16]. Recently, tannins as anti-herpetic agents have been of special interest. The literature provides a lot of evidence that different types of ellagitannins show anti-herpesvirus activity [16-20].
Castalagin demonstrated the highest anti-HSV-1 activity among the tested ellagitannins, attaining efficacy equal to acyclovir [13, 21]. Thus, we used castalagin to study the mode of anti-herpesvirus action of these ellagitannin compounds. Castalagin demonstrated the highest anti-HSV-1 activity among the tested ellagitannins, attaining efficacy equal to acyclovir [13, 21]. Thus, we used castalagin to study the mode of anti-herpesvirus action of these ellagitannin compounds.
Materials and Methods
Cells
Monolayer cultures of Madin-Darbey bovine kidney (MDBK) cells (National Bank for Industrial Microorganisms and Cell Cultures, Sofia, Bulgaria) were grown in DMEM medium containing 10% fetal bovine serum Gibco BRL, USA, supplemented with 10 mM HEPES buffer (Merck, Germany) and antibiotics (penicillin 100 IU/ml, streptomycin 100 μg/ml) in CO2 incubator (HERA cell 150, Heraeus, Germany) at 37 °C/5% CO2.
Virus
Herpes simplex virus type 1, Victoria strain (HSV-1) was received from Prof. S. Dundarov (National Center of Infectious and Parasitic Diseases, Sofia). The virus was replicated in monolayer MDBK cells in a maintenance solution DMEM Gibco BRL, Paisley, Scotland, UK, plus 0.5% bovine fetal serum Gibco BRL, Scotland, UK. Virus assay was based on cytopathic effect measurement following the end-point dilution method of Reed and Muench [22]. Virus titer was expressed as 50% cell culture infectious dose per milliliter (CCID50/ml).
Compound tested
Castalagin is a nonahydroxytriphenoyl-containing C-glucosidic ellagitannin extracted from powdered pedunculate oak (i.e., Quercus robur) heartwood. The substance was purified as previously described [21], dissolved in distilled water to a concentration of 0.01 M and then diluted in DMEM to the required concentration.
Virucidal assay
Two experimental schemes were applied. In the first scheme, equal volumes of virus (105 CCID50) and castalagin at 10 µM (maximal nontoxic concentration, MNC) were mixed. Samples were stored for 15, 30, 60, 90, and 120 min at room temperature. In the second scheme, samples containing virus (105 CCID50) mixed in equal volumes with various concentrations of castalagin, 10 µM, 1 µM, 0.1 µM, 0.01 µM, were incubated for 15, 30, 60, 120, and 150 min at 37 ºC. Then, the residual infectivity in all samples was recorded using the end-point dilution method. The data obtained were compared with control virus samples containing equal volumes of virus suspension and maintenance medium and incubated at the aforementioned intervals: Δlog was determined.
Virus attachment assay
MDBK cell monolayers in 24-well cell culture plates (prechilled at 4 ºC) were inoculated with 103 or 104 CCID50 of HSV-1 for adsorption at 4 ºC and treated in parallel with 10 µM (MNC) or lower concentrations of castalagin. At different time intervals, cells were washed with PBS to remove both the compound and the unattached virus then overlaid with maintenance medium and incubated at 37 ºC for 24 h. Following three rounds of freezing and thawing, the infectious virus titer of each sample was determined using the end-point dilution method. Each sample was prepared in triplicate.
Time-of-addition study
MDBK cells monolayers at a density of 1 × 105 in 24-well cell culture plates were infected with 1 × 105 CCID50 HSV-1 per well. Castalagin was added into wells at different postinfection time intervals. After 12, 15, 18, and 24 h, samples were frozen and thawed three times. The infectious virus titer of each sample was determined using the end-point dilution method. Each sample was prepared in triplicate.
Results
Effect on extracellular virus
The direct effect of castalagin on extracellular HSV-1 virions was investigated. As seen in table 1, castalagin effect on HSV-1 suspension was markedly time dependent at room temperature: it was significant after 30 min of contact (Δlog = 1.7) and attained a Δlog of 2.8 after 150 min.
Incubation at 37 oC substantially strengthened castalagin virucidal effect (Table 1). At 30 min of contact, a marked effect was found at concentrations of 10 μM (Δlog = 1.8) and 1 μM (Δlog = 1.66). Increasing contact time to 60 min had a significant virucidal effect at 0.1 μM (Δlog = 1.7) and even at 0.01 μM (Δlog = 1.5). The strongest effect (Δlog = 3.13) at 37 °C was observed at the 10 μM concentration.
Effect on virion adsorption
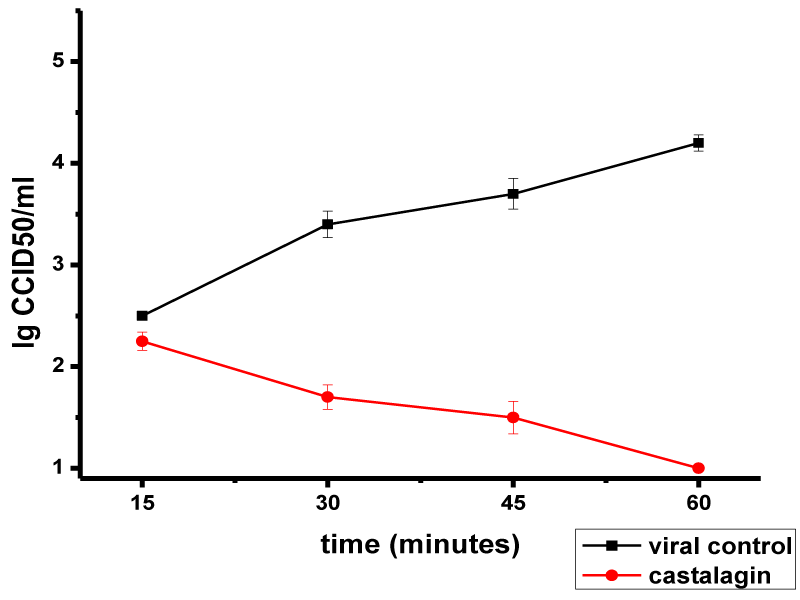
Castalagin effect on the attachment of HSV-1 virions to MDBK cells is presented in Figure 1. No effect on HSV-1 adsorption was observed in the first 15 min of exposure to castalagin (Δlog = 0.25). An inhibitory effect was manifested at 30 min (Δlog = 1.7), and by increasing in the duration of action, the number of adsorbed HSV-1 virions on the cell decreased: Δlog values of 2.2 and 3.2 were recorded at 45 min and 60 min, respectively.
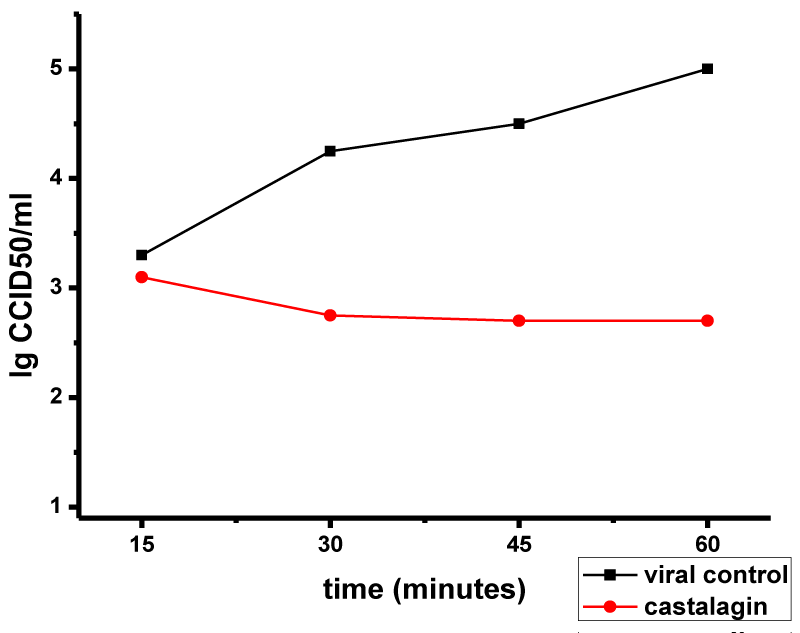
We also investigated the effect of castalagin on virus adsorption at a higher viral inoculation dose (10,000 CCID50/ml, multiplicity of infection of 0.025; Figure 2). Some effect (Δlog = 1.5) was found at 30 min, attaining a Δlog of 1.8 at 45 min. The strongest effect, Δlog = 2, was found at 60 min. Thus, castalagin had less effect on HSV-1 adsorption at a higher viral dose (MI = 0.025) than at a lower viral dose (MI = 0.0025).
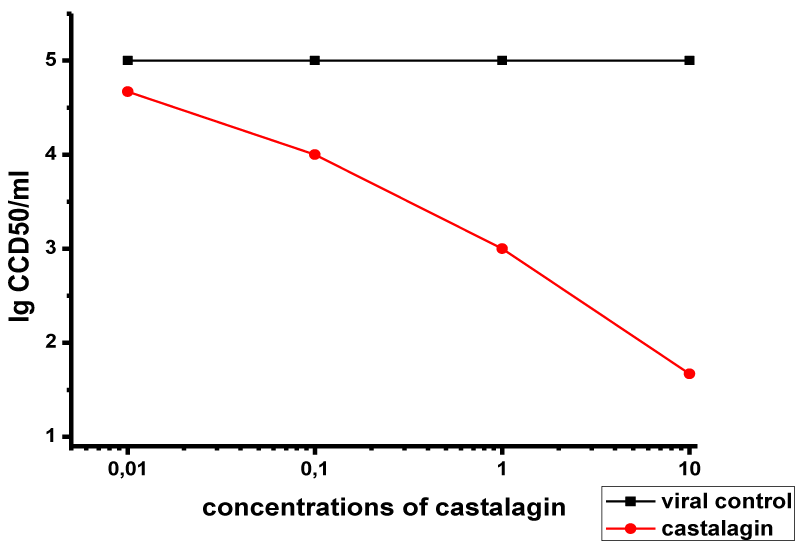
The effects of various concentrations of castalagin on viral adsorption for 60 min are presented in Figure 3. The compound had a marked effect at concentrations of 10 μM (Δlog = 3.2) and 1 μM (Δlog = 2). At 0.1 μM concentration, the effect was markedly weaker (Δlog = 1), and it was absent at 0.01 μM concentration.
These results show that castalagin effect on HSV-1 virion adsorption is dependent on both the concentration of castalagin and the dose of the virus.
Effect on the virus growth curve (time-of-addition study)
Castalagin effect on HSV-1 (ACV-sensitive Victoria strain) replication was studied by following the one-step growth cycle setup in the time-of-addition study (Table 2). The effect was strongest when castalagin was added immediately after virus adsorption (Time 0) or at 3 h postinfection. Adding castalagin at 6 h and 12 h produced certain, but weaker, inhibitory effects. When the compound was added later (18–24 h), no statistically significant inhibitory effect was recorded. Evidently, castalagin affects the earlier stages of the viral replication cycle.
Discussion
In previous studies, we have shown that castalagin possesses selective activity against the replication of ACV-susceptible strains of HSV-1and HSV-2, and its activity is even higher than that of ACV on the HSV-1 strain [13]. The combination of castalagin with ACV manifested a pronounced synergicity. Moreover, when tested against the replication of ACV-resistant strains of HSV-1and HSV-2, it again showed remarkable activity. And in combination with ACV, it again demonstrated a significant synergistic effect [21].
The synergistic character of the effects of castalagin and ACV combinations on HSV-1 and HSV-2 replication indicates that ACV and castalagin have different mechanisms of action (different targets) in HSV-1 and HSV-2 replication cycles.
It is known that ACV targets the TK (+) herpesvirus-encoded DNA polymerase [23]. However, the mechanism of anti-herpesvirus action for castalagin and related substances has not been studied in detail. There is evidence that the ellagitannin eugeniin anti-herpesvirus activity against HSV-1 and HSV-2 inhibits both viral DNA polymerase activity and the synthesis of late proteins [14]. The ellagitannins eugeniflorin D1 and D2 manifested inhibitory effects on Epstein-Barr virus (EBV) DNA polymerase [24]. Another C-glucosidic ellagitannin, cowaniin, showed an inhibitory effect on the EBV early antigen [25]. Some ellagitannins have been shown to inhibit the late stages of viral replication [26,27]. Our experiments regarding the influence of castalagin on adsorption of HSV-1 virions demonstrated a suppression effect after 30 minutes. Literary data from testing other ellagitannin effects on HSV adsorption showed that the ellagitannin casuarinin manifested a marked inhibition of virion adsorption and penetration in the cell [26]. Two other hydrolyzable tannins, chebulagic acid and punicalagin, inactivated the virus particle; because their targets are HSV-1 glycoproteins, they can prevent virus adsorption and penetration as well as virus transition from cell to cell and secondary infection [16].
The results obtained by following the influence of extracellularly applied castalagin on HSV-1 virions showed that castalagin exhibits a direct virucidal effect after 30 minutes, an effect that increases as contact time lengthens. Virucidal effects of excoecarianin, casuarinin, and geraniin have been established as well [28-32].
The time-of-addition study using an HSV-1 one-step virus growth experimental setup and measuring infectious virus formation established that the earliest period (0–3 h) post virus adsorption is the most susceptible to castalagin effect. Most likely, the compound sensitivity period partially includes the next period, 6–12 h. However, the late time interval, 18–24 h, was unsusceptible to castalagin, thus excluding the late phase of HSV-1 virion assembly.
Our results suggest that this study could be considered as an initial step in determining the mechanism of anti-herpesvirus action for the ellagitannin castalagin. Establishing the viral target of castalagin effect remains an open issue that requires further molecular genetic investigation.
- Howard CR, Fletcher NF (2012) Emerging virus diseases: can we ever expect the unexpected? Emerg Microbes Infect 1: e46. Link: https://goo.gl/a8vKhg
- Momméja-Marin H, Lafaurie M, Scieux C, Galicier L, Oksenhendler E, et al. (2003) Herpes simplex virus type 2 as a cause of severe meningitis in immunocompromised adults. Clinical Infect Dis 37: 1527–1533. Link: https://goo.gl/mXWXjb
- Kimberlin DW (2007) Herpes simplex virus of the newborn. Seminars in Perinatology 32: 19-25. Link: https://goo.gl/vCsjtB
- Corey L, Wald A (2009) Maternal and Neonatal Herpes Simplex Virus Infections. J Engl J Med 361: 1376-1385. Link: https://goo.gl/uJ3iU3
- Elion GB, Rideout JL, De Miranda P, Collins P, Bauer DJ (1975) Biological activities of some purine arabinosides. Ann NY Acad Sci 255: 468-480. Link: https://goo.gl/92NhbJ
- Piret J, Boivin G (2011) Resistance of herpes simplex viruses to nucleoside analogues: mechanisms, prevalence, and management. Antimicrob Agents Chemother 55: 459–472. Link: https://goo.gl/L6NYph
- De Clercq E (2013) Antivirals: past, present and future. Biochem Pharmacol 85: 727–744. Link: https://goo.gl/rkdyFD
- Toriyama K, Inoue T, Suzuki T, Kobayashi T, Ohashi Y (2014) Necrotizing keratitis caused by acyclovir-resistant herpes simplex virus. Case Rep Ophthalmol 5 (3): 325–328. Link: https://goo.gl/yfHG84
- Jiang Y-C, Feng H, Lin Y-C, Guo X-R (2016) New strategies against drug resistance to herpes simplex virus. Int J Oral Science 8: 1–6. Link: https://goo.gl/tJApHn
- Quideau S, Feldman KS (1996) Ellagitannin Chemistry. Chem Rev 96: 475-503. Link: https://goo.gl/pFrXdk
- Quideau S (Ed.) (2009) Chemistry and Biology of Ellagitannins, An Underestimated Class of Bioactive Plant Polyphenols. World Scientific Publishing/Imperial College Press: Singapore. Link: https://goo.gl/AC1Ln6
- Quideau S, Deffieux D, Douat-Casassus C, Pouységu L (2011) Plant Polyphenols –Chemical Properties, Biological Activities, and Synthesis. Angew Chem Int Ed 50: 586-621. Link: https://goo.gl/uVHWJZ
- Vilhelmova N, Jacquet R, Quideau S, Stoyanova A, Galabov AS (2011) Three-dimensional analysis of combination effect of ellagitannins and acyclovir on herpes simplex virus types 1 and 2. Antiviral Res 89: 174-181. Link: https://goo.gl/kezDF6
- Kurokawa M, Hozumi T, Basnet P, Nakano M, Kadota S, et al. (1998) Purification and characterization of eugeniin as an anti-herpesvirus compound from Geum japonicum and Syzygium aromaticum. J Pharmacol Exp Ther 284: 728-735. Link: https://goo.gl/pkXLWb
- Grywalska E, Markowicz J, Grabarczyk P, Pasiarski M, Roliński J (2013) Epstein-Barr virus-associated lymphoproliferative disorders. Post Hig Med Dośw 67: 481-490. Link: https://goo.gl/2gy34e
- Lin L-T, ChenT-Y, Chung CY, Noyce RS, Grindley TB, et al. (2011) Hydrolyzable tannins (chebulagic avid and punicalagin) target viral glycoprotein-glycosaminoglycan interactions to inhibit herpes simplex virus 1 entry and cell-to-cell spread. J Virol 85: 4386-4398. Link: https://goo.gl/GxuYFd
- Kurokawa M, Hozumi T, Tsurita M, Kadota S, NambaT, Shiraki K (2001) Biological characterization of eugeniin as an anti-herpes simplex virus type 1 compound in vitro and in vivo. J Pharmacol Exp Ther 297: 372-379. Link: https://goo.gl/HAUq4c
- Chattopadhyay D, Das S, Chakraborty S, Bhattacharya SK (2010) Ethnomedicines for the development of anti-herpesvirus agents. Ethnomedicine: Source of Complem Ther 117-147. Link: https://goo.gl/UgM832
- Mishra KP, Sharma N, Diwaker D, Ganju L, Singh SB (2013) Plant derived antivirals: a potential source of drug development. J Virol Antivir Res 2: 2. Link: https://goo.gl/AqbMwf
- Houston DMJ, Bugert J., Denyer SP, Heard CM (2017) Anti-inflammatory activity of Punica granatum L. (Pomegranate) rind extracts applied topically to ex vivo skin. Eur J Pharmac Biopharmac 112: 30-37. Link: https://goo.gl/ad8c77
- Vilhelmova-Ilieva N, Jacquet R, Quideau S, Galabov AS (2014) Ellagitannins as synergists of ACV on the replication of ACV-resistant strains of HSV 1 and 2. Antiviral Res 110: 104-114. Link: https://goo.gl/SUt6AE
- Reed LJ, Muench H (1938) A simple method of estimating fifty percent endpoints. Amer J Hyg 27: 493-497. Link: https://goo.gl/jxm3uw
- Elion GB (1982) Mechanism of action and selectivity of acyclovir. Am J Med 73: 7-15. Link: https://goo.gl/xkMHRG
- Lee MH, Chiou JF, Yen KY, Yang LL (2000). EBV DNA polimerase inhibition of tannins from Eugenia uniflora. Cancer Lett 154: 131-136. Link: https://goo.gl/N3cxgH
- Ito H, Miyake M, Nishitani E, Miyashita K, Yoshimura M, et al. (2007) Cowaniin, a C-Glucosidic Ellagitannin Dimer Linked through Catechin from Cowania mexicana. Chem Pharm Bull 55: 492-494. Link: https://goo.gl/jwQk3Q
- Cheng HY, Lin II, LinTI (2002) Antiherpes simplex virus type 2 activity of casuarinin from the bark of Terminalia arjuna Linn. Antiviral Res 55: 447-455. Link: https://goo.gl/CrCgiE
- Cheng H-Y, Lin T-C, Yanf C-M, Wang K-C, Lin L-T,Lin C-C(2004) Putranjivain A from Euphorbia jolkini inhibits both virus entry and late stage replication of herpes simplex virus type 2 in vitro. J Antimicrob Chemother 53: 577-583. Link: https://goo.gl/4Y4qEa
- Cheng HY, Yang CM, Lin TC, Lin LT, Chiang LC, (2011) Excoecarianin, isolated from Phyllanthus urinaria Linnea, inhibits herpes simplex virus type 2 infection through inactivation of viral particles. Evidence-Based Complementary & Alternative Med 2011: 259103. Link: https://goo.gl/xjFDuT
- Notka F, Meier G, Wagner R (2004) Concerted inhibitory activities of Phyllanthus amarus on HIV replication in vitro and ex vivo. Antiviral Res 64: 93–102. Link: https://goo.gl/ev6bXB
- Notka F, Meier G, Wagner R (2003) Inhibition of wild-type human immunodeficiency virus and reverse transcriptase inhibitor-resistant variants by Phyllanthus amarus. Antiviral Res 58: 175–186. Link: https://goo.gl/q7gRWy
- Yang C, Cheng H, LinT, Chiang L, Lin C (2007) The in vitro activity of geraniin and 1,3,4,6-tetra-O-galloyl-β-d-glucose isolated from Phyllanthus urinaria against herpes simplex virus type 1 and type 2 infection. J Ethnopharmacol 110: 555–558. Link: https://goo.gl/T5qeN4
- Li J, Huang H, Zhou W, Feng M, Zhou P (2008) Anti-hepatitis B virus activities of Geranium caroliniamum L. extracts and identification of the active compounds. Biol Pharm Bull 3: 743–747. Link: https://goo.gl/9fmJ2z
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
